Abstract
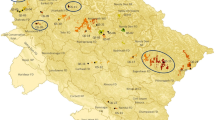
Quercus ilex L. (holm oak) is a wind-pollinated, sclerophyllous tree that copes with the environmental variability of the Mediterranean climate and that displays flexible ecophysiological adaptability in relation to hydric and thermic stresses. The holm oak dominates Mediterranean woodlands on both acidic and calcareous soils and has been exposed to management (dehesas) for thousands of years. Both protected areas and glacial refugia are supposed to preserve a substantial fraction of the genetic diversity of Iberian species. Genetic diversity was examined for 68 populations sampled throughout Spain using ptDNA SNPs, ptDNA microsatellites, and primarily nuclear AFLPs. Protected populations did not significantly differ from nonprotected populations by any of the measures of levels of genetic diversity. The three-level hierarchical AMOVA indicated that a low number of protected populations harbor most of the species’ genetic diversity. In addition, we found no evidence from either ptDNA or AFLP variation to support that populations from putative glacial refugia are divergent genetic groups as expected during isolation. Outcrossing, anemophilous long-distance pollen dispersal, acorn transport by animals, tree reliance, and habitat availability in Spain probably played a primarily role in homogenizing allele frequency among populations. This result leads us to suggest that extensive gene flow has been prevalent across Spanish populations. We conclude that glacial refugia have not been essential to maintain the neutral genetic makeup of Q. ilex. Nevertheless, conservation of the holm oak in protected areas ensures protection of the species’ genetic diversity, the most widespread woodland ecosystem in Iberia and indirectly the four iconic, endangered animal species (black stork, cinereous vulture, Iberian lynx, western imperial eagle).


Similar content being viewed by others
References
Albert A, Jahandiez E (1908) Catalogue des plantes vasculaires du departement du Var. Paris
Aldrich PR, Cavender-Bares J (2011) Quercus. In: Kole C (ed) Wild crop relatives: genomic and breeding resources: forest trees. Springer Science & Business Media, pp 89–129
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Barbero M, Loisel R, Quézel P (1992) Biogeography, ecology and history of Mediterranean Quercus ilex ecosystems. Vegetatio 99–100:19–34
Bird A (2007) Perceptions of epigenetics. Nature 447:396–398
Bozzano M, Turok J (2003) Mediterranean Oaks Network, report of the second meeting, 2–4 May 2002—Gozo, Malta. International Plant Genetic Resources Institute, Rome, Italy
Bradshaw AD (1965) Evolutionary significance of phenotypic plasticity. Adv Genet 13:115–155
Bussotti F, Pollastrini M, Holland V, Brüggemann W (2015) Functional traits and adaptive capacity of European forests to climate change. Environ Exp Bot 111:91–113
Carnicer J, Coll M, Pons X, Ninyerola M, Vayreda J, Peñuelas J (2014) Large-scale recruitment limitation in Mediterranean pines: the role of Quercus ilex and forest successional advance as key regional drivers. Global Ecol Biogeogr 23:371–384
Coart E, Van Glabeke S, Petit R, Van Bockstaele E, Roldán-Ruiz I (2005) Range wide versus local patterns of genetic diversity in hornbeam (Carpinus betulus L.). Conserv Genet 6:259–273
Coelho AC, Lima MB, Neves D, Cravador A (2006) Genetic diversity of two Evergreen Oaks [Quercus suber (L.) and Quercus ilex subsp. rotundifolia (Lam.)] in Portugal using AFLP markers. Silvae Genet 55:105–118
Comes HP, Kadereit JW (1998) The effect of Quaternary climatic changes on plant distribution and evolution. Trends Plant Sci 3:432–438
Craft KJ, Ashley MV (2007) Landscape genetic structure of bur oak (Quercus macrocarpa) savannas in Illinois. For Ecol Manag 239:13–20
Díaz M, Pulido FJ, Marañón T (2003) Diversidad biológica y sostenibilidad ecológica y económica de los sistemas adehesados. Ecosistemas 3:http://www.aeet.org/ecosistemas/033/investigacion034.htm
do Amaral J (1990) Quercus L. In: Castroviejo S, Laínz M, López G, Montserrat P, Muñoz-Garmendia F, Paiva J, Villar L (eds) Flora Ibérica, vol 2. Consejo Superior de Investigaciones Ciéntificas, Madrid, pp 19–20
Dodd RS, Kashani N (2003) Molecular differentiation and diversity among the California red oaks (Fagaceae; Quercus section Lobatae). Theor Appl Genet 107:884–892
Ducousso A, Michaud H, Lumaret R (1993) Reproduction and gene flow in the genus Quercus L. Ann Sci For 50:s91–s106
Dumolin S, Demesure B, Petit RJ (1995) Inheritance of chloroplast and mitochondrial genomes in pedunculate oak investigated with an efficient PCR method. Theor Appl Genet 91:1253–1256
Ehrich D (2006) AFLPdat: a collection of R functions for convenient handling of AFLP data. Mol Ecol Notes 6:603–604
EUROPARC-España (2012) Anuario 2011 del estado de las áreas protegidas en España. Fundación Fernando González Bernáldez, Madrid
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
FAO (1989) Plant genetic resources. Their conservation in situ for human use. Rome
FAO, DFSC, IPGRI (2001) Forest genetic resources conservation and management, vol 2, In managed natural forests and protected areas (in situ). International Plant Genetic Resources Institute, Rome, Italy
Felicísimo AMC, Muñoz J, Villalba CJ, Mateo RG (2011) Impactos, vulnerabilidad y adaptación al cambio climático de la biodiversidad española, 2nd edn, Flora y vegetación. Oficina Española de Cambio Climático, Ministerio de Medio Ambiente y Medio Rural y Marino, Madrid
Frankel OH, Bennett E (1970) Genetic resources in plants—their exploration and conservation. Blackwell, Oxford
Frankham R (2005) Genetics and extinction. Biol Conserv 126:131–140
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Gaudet M, Fara A-G, Sabatti M, Kuzminsky E, Mugnozza GS (2007) Single reaction for SNP genotyping on agarose gel by allele-specific PCR in black poplar (Populus nigra L.). Plant Mol Biol Report 25:1–9
Gimeno T, Pías B, Lemos-Filho JP, Valladares F (2009) Plasticity and stress tolerance override local adaptation in the responses of Mediterranean holm oak seedlings to drought and cold. Tree Physiol 29:87–98
Gimeno TE, Pías B, Lemos-Filho JP, Valladares F (2008) Plasticity and stress tolerance override local adaptation in the responses of Mediterranean holm oak seedlings to drought and cold. Tree Physiol 29:87–98
Hamrick JL, Godt MJW (1989) Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weiss BS (eds) Plant population genetics, breeding and genetic resources. Sinauer, Sunderland, pp 43–63
Hewitt GM (1996) Some genetic consequences of ice ages, and their role in divergence and speciation. Bot J Linn Soc 58:247–276
Hewitt GM (2001) Speciation, hybrid zones and phylogeography—or seeing genes in space and time. Mol Ecol 10:537–549
Holling CS (1996) Surprise for science, resilience for ecosystems, and incentives for people. Ecol Appl 6:733–735
IPCC (2007) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change 2007. Cambridge University Press, Cambridge
Jimenez P, Diaz-Fernandez PM, Iglesias S et al (2009) Strategy for the conservation and sustainable use of Spanish forest genetic resources. Investigacion Agraria-Sistemas Y Recursos Forestales 18:13–19
Jiménez P, López de Heredia U, Collada C, Lorenzo Z, Gil L (2004) High variability of chloroplast DNA in three Mediterranean evergreen oaks indicates complex evolutionary history. Heredity 93:510–515
Kalinowski ST (2005) HP-RARE 1 0: a computer program for performing rarefaction on measures of allelic richness. Mol Ecol Notes 5:187–189
Kelleher CT, Hodkinson TR, Douglas GC, Kelly DL (2005) Species distinction in Irish populations of Quercus petraea and Q. robur: morphological versus molecular analyses. Ann Bot 96:1237–1246
Koskela J, Vinceti B, Dvorak W, Bush D, Dawson IK, Loo J et al (2014) Utilization and transfer of forest genetic resources: a global review. For Ecol Manag 333:22–34
Larcher W (1960) Transpiration and photosynthesis of detached leaves and shoots of Quercus pubescens and Q. ilex during desiccation under standard conditions. Bull Res Counc Isr Sect E Exp Med 8D:213–224
Larcher W, Mair B (1969) Die Temperaturresistenz als ökophysiologisches Konstitutionsmerkmal Quercus ilex und andere Eichenarten des Mittelmeergebietes. Oecol Plant 4:347–376
Lefèvre F et al (2013) Dynamic conservation of forest genetic resources in 33 European countries. Conserv Biol 27:373–384
Leinonen T, O’Hara RB, Cano JM, Merilä J (2008) Comparative studies of quantitative trait and neutral marker divergence: a meta-analysis. J Evol Biol 21:1–17
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
López de Heredia U, Carrión JS, Jiménez P, Collada C, Gil L (2007a) Molecular and palaeoecological evidence for multiple glacial refugia for evergreen oaks on the Iberian Peninsula. J Biogeogr 34:1505–1517
López de Heredia U et al (2007b) Multi-marker phylogeny of three evergreen oaks reveals vicariant patterns in the western Mediterranean. Taxon 56:1209–1220
Lumaret R, Mir C, Michaud H, Raynald V (2002) Phylogeographical variation of chloroplast DNA in holm oak (Quercus ilex L.). Mol Ecol 11:2327–2336
Lynch M, Milligan BG (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99
Maldonado J, Sainz H, Sánchez R, Xandri P (2001) Distribución y estado de conservación de los bosques españoles: Un análisis de las carencias en la red de territorios protegidos. In: Plana E, Campodrón J (eds) Conservación de la biodiversidad y gestión forestal. Universidad de Barcelona, Barcelona, pp 101–117
Marañón T (1986) Plant species richness and canopy effect in the savanna-like “dehesa” of SW-Spain. Ecol Medit 12:131–141
Maxted N, Iriondo JM, Dulloo ME, Lane A (2008) Introduction: the integration of PGR conservation with protected area management. In: Iriondo JM, Maxted N, Dulloo ME (eds) Conserving plant genetic diversity in protected areas. CAB International, Wallingford, pp 1–22
Médail F, Diadema K (2009) Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J Biogeogr 36:1333–1345
Michaud H, Lumaret R, Romane F (1992) Variation in the genetic structure and reproductive biology of holm oak populations. Vegetatio 99–100:107–113
Mousseau TA, Sinervo B, Endler JA (1999) Adaptive genetic variation in the wild. Oxford University Press, Oxford
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci 76:5269–5273
Ortego J, Bonal R, Muñoz A (2010) Genetic consequences of habitat fragmentation in long-lived tree species: the case of the Mediterranean holm oak (Quercus ilex, L.). J Hered 101:717–726
Palmberg-Lerche C (2007) Forest biological diversity and forest tree and shrub genetic resources: concepts, conservation strategies, priorities and values. Nature & Faune 22:21–28
Parsons JJ (1962) The acorn-hog economy of the oak woodlands of southwestern Spain. Geogr Rev 52:211–235
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Peguero-Pina JJ, Sancho-Knapik D, Barrón E, Camarero JJ, Vilagrosa A, Gil-Pelegrín E (2014) Morphological and physiological divergences within Quercus ilex support the existence of different ecotypes depending on climatic dryness. Ann Bot 114:301–313
Petit R, El Mousadik A, Pons O (1998) Identifying populations for conservation on the basis of genetic markers. Conserv Biol 12:844–855
Petit RJ et al (2003) Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300:1563–1565
Petit RJ et al (2002) Chloroplast DNA variation in European white oaks: phylogeography and patterns of diversity based on data from over 2600 populations. For Ecol Manag 156:5–26
Read J, Sanson GD (2003) Characterizing sclerophylly: the mechanical properties of a diverse range of leaf types. New Phytol 160:81–99
Rico L, Ogaya R, Barbeta A, Peñuelas J (2014) Changes in DNA methylation fingerprint of Quercus ilex trees in response to experimental field drought simulating projected climate change. Plant Biol 16:419–427
Saccheri I, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I (1998) Inbreeding and extinction in a butterfly metapopulation. Nature 392:491–494
Sainz H, Sánchez de Dios R, García-Cervigón A (2010) La cartografía sintética de los paisajes vegetales españoles: una asignatura pendiente en geobotánica. Ecología 23:249–272
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schönswetter P, Tribsch A (2005) Vicariance and dispersal in the alpine perennial Bupleurum stellatum L. (Apiaceae). Taxon 54:725–732
Sebastiani F, Carnevale S, Vendramin GG (2004) A new set of mono- and dinucleotide chloroplast microsatellites in Fagaceae. Mol Ecol Notes 4:259–261
Shafer ABA, Cullingham CI, Cote SD, Coltman DW (2010) Of glaciers and refugia: a decade of study sheds new light on the phylogeography of northwestern North America. Mol Ecol 19:4589–4621
Sherwin WB, Moritz C (2000) Managing and monitoring genetic erosion. In: Young AG, Clarke GM (eds) Genetics, demography and viability of fragmented populations. Cambridge University Press, Cambridge, pp 9–34
Shiran B, Mashayekhi S, Jahanbazi H, Soltani A, Bruschi P (2011) Morphological and molecular diversity among populations of Quercus brantii Lindl. in western forest of Iran. Plant Biosyst 145:452–460
Swofford D (2002) PAUP*. Phylogenetic analysis using parsimony (*and other methods), 4th edn. Sinauer, Sunderland
Taberlet P, Fumagalli L, Wust-Saucy A, Cosson J (1998) Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol 7:453–464
Tellería JL (2001) Passerine bird communities of Iberian dehesas: a review. Anim Biodivers Conserv 24:67–78
Újvária B, Madsenb T, Kotenkod T, Olssone M, Shinec R, Wittzellf H (2002) Low genetic diversity threatens imminent extinction for the Hungarian meadow viper (Vipera ursinii rakosiensis). Biol Conserv 105:127–130
UNEP-WCMC (2008) Guidelines for applying protected area management categories. In: Dudley N (ed) About protected areas. IUCN, Geneva, pp 8–9
Vekemans X, Beauwens T, Lemaire M, Roldan-Ruiz I (2002) Data from amplified fragment length polymorphism (AFLP) markers show indication of size homoplasy and of a relationship between degree of homoplasy and fragment size. Mol Ecol 11:139–151
Vernesi C, Rocchini D, Pecchioli E, Neteler M, Vendramin GG, Paffetti D (2012) A landscape genetics approach reveals ecological-based differentiation in populations of holm oak (Quercus ilex L.) at the northern limit of its range. Bot J Linn Soc 107:458–467
Vos P et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Weising K, Gardner RC (1999) A set of conserved PCR primers for the analysis of simple sequence repeat polymorphisms in chloroplast genomes of dicotyledonous angiosperms. Genome 42:9–19
Weiss S, Ferrand N (2007) Phylogeography of southern European refugia. Springer Science & Business Media
Wolf P, Doche B, Gielly L, Taberlet P (2004) Genetic structure of Rhododendron ferrugineum at a wide range of spatial scales. J Hered 95:301–308
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trends Ecol Evol 11:413–418
Zhivotovsky LA (1999) Estimating population structure in diploids with multilocus dominant DNA markers. Mol Ecol 8:907–913
Acknowledgments
The authors thank J. Arroyo, J. Bastida, J. Belliure, J. Camarero, M. Díaz, O. Fiz, P. García-Fayos, C. García Verdugo, C.M. Herrera, O. Lozoya, J. Martínez, V. Mirre, J. Pausas, F. Pulido, A. Tribsch and F. Valladares for field assistance; J. Fernández (Quantum Gis) for analytical assistance; and H. Sainz and R. Sánchez de Dios for providing the Quercus ilex distribution map layer. This research was supported by Fundación Biodiversidad through the project “Los parques nacionales españoles como reserva genética para la encina (Quercus ilex), el alcornoque (Quercus suber) y el acebuche (Olea europaea)” to PV and by the Spanish Ministry of Economy and Competitiveness through a Juan de la Cierva fellowship to BG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by A. Kremer
Data archiving statement
We have submitted the PAMSA SNPs/AFLPs datasets to http://dendrome.ucdavis.edu/TreeGenes database (accession number: TGDR042).
This article is part of the Topical Collection on Germplasm Diversity
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 12086 kb)
Rights and permissions
About this article
Cite this article
Guzmán, B., Rodríguez López, C.M., Forrest, A. et al. Protected areas of Spain preserve the neutral genetic diversity of Quercus ilex L. irrespective of glacial refugia. Tree Genetics & Genomes 11, 124 (2015). https://doi.org/10.1007/s11295-015-0950-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-015-0950-2