Abstract
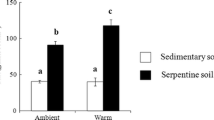
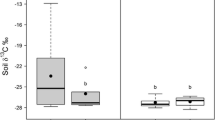
Calcium (Ca)-rich food can increase feeding of Lumbricidae. Earthworms can be genetically differentiated at a small spatial scale and acclimatize to the local environment during growth. Soil feeding and subsequent cast production by earthworms affects soil N mineralization. Here, we hypothesized that soil feeding and subsequent cast production by Lumbricidae species increases with high soil Ca content and that this increase is stronger in worms from high-Ca soil. We also hypothesized that changes in the soil feeding of Lumbricidae species along with the Ca content affects the soil N mineralization via changes in the cast production. Using a geophageous earthworm species (Eisenia japonica) originated from two different Ca environments (calcareous soil and sedimentary soil), we investigated cast production and soil N mineralization in three soils (sedimentary soil, sedimentary soil with Ca addition, and calcareous soil). The soil feeding of E. japonica from both origins did not always increase despite the high soil Ca content. We suggest that both the Ca content and other soil conditions (e.g., soil C:N) might be major factors in increasing soil feeding by E. japonica. Furthermore, the influence of Ca addition on cast production varied according to the earthworm origin. As expected, these differences in cast production are linked to soil N mineralization (especially nitrification). In summary, our study suggests that the acclimatization and/or adaptation of Lumbricidae species to local environmental factors not only soil Ca content explains spatially heterogeneous soil N mineralization in forest soil.



Similar content being viewed by others
References
Auclerc A, Nahmani J, Huguier P, Capowiez Y, Aran D, Guérold F (2011) Adapting ecotoxicological tests based on earthworm behavior to assess the potential effectiveness of forest soil liming. Pedobiologia (Jena) 54:63–68
Berg M, De Ruiter P, Didden W, Janssen M, Schouten T, Verhoef H (2001) Community food web, decomposition and nitrogen mineralisation in a stratified Scots pine forest soil. Oikos 94:130–142
Blanchart E (1992) Restoration by earthworms (megascolecidae) of the macroaggregate structure of a destructured savanna soil under field conditions. Soil Biol Biochem 24:1587–1594
David JF, Gillon D (2009) Combined effects of elevated temperatures and reduced leaf litter quality on the life-history parameters of a saprophagous macroarthropod. Glob Chang Biol 15:156–165
Dupont L, Gresille Y, Richard B, Decaëns T, Mathieu J (2015) Dispersal constraints and fine-scale spatial genetic structure in two earthworm species. Biol J Linn Soc 114:335–347
Edwards CA (2004) Earthworm ecology, 2nd edn. CRC, Florida
Edwards CA, Bohlen PJ (1996) Biology and ecology of earthworms, 3rd edn. Chapman and Hall, London
Hillebrand H, Borer ET, Bracken MES, Cardinale BJ, Cebrian J, Cleland EE, Elser JJ, Gruner DS, Stanley Harpole W, Ngai JT, Sandin S, Seabloom EW, Shurin JB, Smith JE, Smith MD (2009) Herbivore metabolism and stoichiometry each constrain herbivory at different organizational scales across ecosystems. Ecol Lett 12:516–527
Holdsworth AR, Frelich LE, Reich PB (2012) Leaf litter disappearance in earthworm-invaded Northern Hardwood forests: role of tree species and the chemistry and diversity of litter. Ecosystems 15:913–926
Ishizuka K (2014) Pictorial book of earthworm. National Rural Education Association, Tokyo
Kawaguchi T, Kyoshima T, Kaneko N (2011) Mineral nitrogen dynamics in the casts of epigeic earthworms (Metaphire hilgendorfi: Megascolecidae). Soil Sci Plant Nutr 57:387–395
Kharin SA, Kurakov AV (2009) Transformation of nitrogen compounds and dynamics of microbial biomass in fresh casts of Aporrectodea caliginosa. Eurasian Soil Sci 42:75–81
Lavelle P, Bignell D, Lepage M, Wolters W, Roger P, Ineson P, Heal OW, Dhillion S (1997) Soil function in a changing world: the role of invertebrate ecosystem engineers. Eur J Soil Biol 33:159–193
Makoto K, Minamiya Y, Kaneko N (2016) Differences in soil type drive the intraspecific variation in the responses of an earthworm species and consequently, tree growth to warming. Plant Soil 404:209–218
Marhan S, Auber J, Poll C (2015) Additive effects of earthworms, nitrogen-rich litter and elevated soil temperature on N2O emission and nitrate leaching from an arable soil. Appl Soil Ecol 86:55–61
Milcu A, Manning P (2011) All size classes of soil fauna and litter quality control the acceleration of litter decay in its home environment. Oikos 120:1366–1370
Nakamura Y (1972) Ecological studies on the Family Lumbricidae from Hokkaido I, ecological distribution. Jpn Soc Appl Entomol Zool 16:18–23
Ott D, Rall BC, Brose U (2012) Climate change effects on macrofaunal litter decomposition: the interplay of temperature, body masses and stoichiometry. Philos Trans R Soc B Biol Sci 367:3025–3032
Scheu S (1987) The influence of earthworms (Lumbricidae) on the nitrogen dynamics in the soil litter system of a deciduous forest. Oecologia 72:197–201
Simon A, Sivasithamparam K (1989) Pathogen-suppression—a case-study in biological suppression of gaeumannomyces-graminis var tritici in soil. Soil Biol Biochem 21:331–337
Springett JA, Syers JK (1984) Effect of pH and calcium content of soil on earthworm cast production in the laboratory. Soil Biol Biochem 16:185–189
Sullivan TJ, Dreyer AP, Peterson JW (2009) Genetic variation in a subterranean arthropod (Folsomia candida) as a method to identify low-permeability barriers in an aquifer. Pedobiologia (Jena) 53:99–105
Toyota A, Hynšt J, Cajthaml T, Frouz J (2013) Soil fauna increase nitrogen loss in tilled soil with legume but reduce nitrogen loss in non-tilled soil without legume. Soil Biol Biochem 60:105–112
Vitousek PM, Hättenschwiler S, Olander L, Allison S (2002) Nitrogen and nature. Ambio 31:97–101
Acknowledgements
We acknowledge Mr. T. Miura in Nakatonbetsu Town and the technical staff of Tesio Experimental Forest of Hokkaido University for their support during the research. We also sincerely thank Dr. K. Takagi, Professor Y. Hashidoko, Ms. R. Isoda and Ms. E. Marumo for their comments on this project. The analysis of soil and earthworm samples was carried out with ICPE-9000 at the OPEN FACILITY, Hokkaido University Sousei Hall. This project is financially supported by JSPS for young researchers (Type B to K.M.) and by the Kuribayashi Ikuei Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kawakami, T., Makoto, K. Does an earthworm species acclimatize and/or adapt to soil calcium conditions? The consequences of soil nitrogen mineralization in forest soil. Ecol Res 32, 603–610 (2017). https://doi.org/10.1007/s11284-017-1473-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-017-1473-0