Abstract
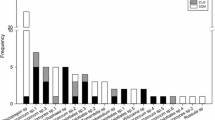
Interactions between trees and ectomycorrhizal fungi are critical to the growth and survival of both partners. However, ectomycorrhizal symbiosis has barely been explored in endangered trees, and no information is available regarding soil spore banks of ectomycorrhizal fungi from forests of threatened trees. Here, we evaluated soil spore banks of ectomycorrhizal fungi from endangered Japanese Douglas-fir (Pseudotsuga japonica) forests using bioassay approaches with congeneric P. menziesii and Pinus densiflora seedlings in combination with molecular identification techniques. Rhizopogon togasawariana was predominant in soil propagule banks and was found in all remaining P. japonica forests when assayed with P. menziesii, while no colonization of this fungus was observed on Pinus seedlings. Given the observed specificity of R. togasawariana for P. menziesii and its phylogenetic position within the Pseudotsuga-specific Rhizopogon lineage, its geographical distribution is likely restricted to the remaining Japanese Douglas-fir forests, indicating a high extinction risk for this fungus as well as its endangered host. Spore banks of R. togasawariana remained highly infective after preservation for 1 year or heat treatment at 70 °C, suggesting an ecological strategy of establishing ectomycorrhizal associations on regenerating Japanese Douglas-fir seedlings after disturbance, as observed in other Rhizopogon–Pinaceae combinations. Therefore, the regeneration of Japanese Douglas-fir seedlings may depend largely on the soil spore banks dominated by R. togasawariana, which has co-evolved with the Japanese Douglas-fir for over 30 million years. More attention must be paid to underground ectomycorrhizal fungi for the conservation of endangered tree species, especially in the era of human-induced mass extinction.




Similar content being viewed by others
References
Amaranthus MP, Li CY, Perry DA (1990) Influence of vegetation type and madrone soil inoculum on associative nitrogen-fixation in Douglas-fir rhizospheres. Can J For Res 20:368–371
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Ashkannejhad S, Horton TR (2006) Ectomycorrhizal ecology under primary succession on coastal sand dunes: interactions involving Pinus contorta, suilloid fungi and deer. New Phytol 169:345–354
Baar J, Horton TR, Kretzer AM, Bruns TD (1999) Mycorrhizal colonization of Pinus muricata from resistant propagules after a stand-replacing wildfire. New Phytol 143:409–418
Barker JS, Simard SW, Jones MD, Durall DM (2013) Ectomycorrhizal fungal community assembly on regenerating Douglas-fir after wildfire and clearcut harvesting. Oecologia 172:1179–1189
Beiler KJ, Durall DM, Simard SW, Maxwell SA, Kretzer AM (2010) Architecture of the wood-wide web: rhizopogon spp. genets link multiple Douglas-fir cohorts. New Phytol 185:543–553
Borcard D, Gillet F, Legendre P (2011) Numerical Ecology with R. Springer, NY
Bruns TD, Peay KG, Boynton PJ, Grubisha LC, Hynson NA, Nguyen NH, Rosenstock NP (2009) Inoculum potential of Rhizopogon spores increases with time over the first 4 year of a 99-year spore burial experiment. New Phytol 181:463–470
Buscardo E, Rodriguez-Echeverria S, Martin MP, De Angelis P, Pereira JS, Freitas H (2010) Impact of wildfire return interval on the ectomycorrhizal resistant propagules communities of a Mediterranean open forest. Fungal Biol 114:628–636
Chuchou M, Grace LJ (1981) Mycorrhizal fungi of Pseudotsuga-menziesii in the north island of New-Zealand. Soil Biol Biochem 13:247–249
Cline ET, Ammirati JF, Edmonds RL (2005) Does proximity to mature trees influence ectomycorrhizal fungus communities of Douglas-fir seedlings? New Phytol 166:993–1009
Collier FA, Bidartondo MI (2009) Waiting for fungi: the ectomycorrhizal invasion of lowland heathlands. J Ecol 97:950–963
Colwell RK, Chao A, Gotelli NJ, Lin SY, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol 5:3–21
Douhan GW, Huryn KL, Douhan LI (2007) Significant diversity and potential problems associated with inferring population structure within the Cenococcum geophilum species complex. Mycologia 99:812–819
FAO (2010) Global forest resources assessment 2010. FAO forestry paper 163. FAO, Rome
Frank JL, Anglin S, Carrington EM, Taylor DS, Viratos B, Southworth D (2009) Rodent dispersal of fungal spores promotes seedling establishment away from mycorrhizal networks on Quercus garryana. Bot Bot 87:821–829
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes–application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Glassman SI, Peay KG, Talbot JM, Smith DP, Chung JA, Taylor JW, Vilgalys R, Bruns TD (2015) A continental view of pine-associated ectomycorrhizal fungal spore banks: a quiescent functional guild with a strong biogeographic pattern. New Phytol 205:1619–1631
Glassman SI, Levine CR, DiRocco AM, Battles JJ, Bruns TD (2016) Ectomycorrhizal fungal spore bank recovery after a severe forest fire: some like it hot. ISME J 10:1228–1239
Grubisha LC, Trappe JM, Molina R, Spatafora JW (2002) Biology of the ectomycorrhizal genus Rhizopogon. VI. re-examination of infrageneric relationships inferred from phylogenetic analyses of ITS sequences. Mycologia 94:607–619
Hagerman SM, Durall DM (2004) Ectomycorrhizal colonization of greenhouse-grown Douglas-fir (Pseudotsuga menziesii) seedlings by inoculum associated with the roots of refuge plants sampled from a Douglas-fir forest in the southern interior of British Columbia. Can J Bot 82:742–751
Hartford RA, Frandsen WH (1992) When it is hot, it is hot… or maybe it’s not! (Surface flaming may not portend extensive soil heating). Int J Wildland Fire 2:139–144
Horton TR, Bruns TD (1998) Multiple-host fungi are the most frequent and abundant ectomycorrhizal types in a mixed stand of Douglas-fir (Pseudotsuga menziesii) and Bishop pine (Pinus muricata). New Phytol 139:331–339
Horton TR, Bruns TD, Parker VT (1999) Ectomycorrhizal fungi associated with Arctostaphylos contribute to Pseudotsuga menziesii establishment. Can J Bot 77:93–102
Horton TR, Molina R, Hood K (2005) Douglas-fir ectomycorrhizae in 40- and 400-year-old stands: mycobiont availability to late successional western hemlock. Mycorrhiza 15:393–403
Huang J et al (2014) Ectomycorrhizal fungal communities associated with Masson pine (Pinus massoniana) and white oak (Quercus fabri) in a manganese mining region in Hunan Province, China. Fungal Ecol 9:1–10
Huang J, Nara K, Zong K, Lian CL (2015) Soil propagule banks of ectomycorrhizal fungi along forest development stages after mining. Microb Ecol 69:768–777
Ishida TA, Nara K, Hogetsu T (2007) Host effects on ectomycorrhizal fungal communities: insight from eight host species in mixed conifer-broadleaf forests. New Phytol 174:430–440
Ishida TA, Nara K, Tanaka M, Kinoshita A, Hogetsu T (2008) Germination and infectivity of ectomycorrhizal fungal spores in relation to their ecological traits during primary succession. New Phytol 180:491–500
IUCN (2016) IUCN red list of threatened species. http://www.iucnredlist.org. Accessed July 2016
Iwaizumi MG, Takahashi M, Isoda K, Austerlitz F (2013) Consecutive 5-year analysis of paternal and maternal gene flow and contributions of gametic heterogeneities to overall genetic composition of dispersed seeds of Pinus Densiflora (Pinaceae). Am J Bot 100:1896–1904
Izzo A, Canright M, Bruns TD (2006) The effects of heat treatments on ectomycorrhizal resistant propagules and their ability to colonize bioassay seedlings. Mycol Res 110:196–202
Jones MD, Durall DM, Harniman SMK, Classen DC, Simard SW (1997) Ectomycorrhizal diversity on Betula papyrifera and Pseudotsuga menziesii seedlings grown in the greenhouse or outplanted in single-species and mixed plots in southern British Columbia. Can J For Res 27:1872–1889
Jones MD, Twieg BD, Ward V, Barker J, Durall DM, Simard SW (2010) Functional complementarity of Douglas-fir ectomycorrhizas for extracellular enzyme activity after wildfire or clearcut logging. Funct Ecol 24:1139–1151
Kinoshita N, Shima I, HIrono M (2004) The characteristic of germination and establishment of pioneer species in fire affected forest (in Japanese). J Jap Soc Rev Technol 30:336–339
Kipfer T, Egli S, Ghazoul J, Moser B, Wohlgemuth T (2010) Susceptibility of ectomycorrhizal fungi to soil heating. Fungal Biol 114:467–472
Kipfer T, Moser B, Egli S, Wohlgemuth T, Ghazoul J (2011) Ectomycorrhiza succession patterns in Pinus sylvestris forests after stand-replacing fire in the Central Alps. Oecologia 167:219–228
Kjoller R, Bruns TD (2003) Rhizopogon spore bank communities within and among California pine forests. Mycologia 95:603–613
Kretzer AM, Dunham S, Molina R, Spatafora JW (2004) Microsatellite markers reveal the below ground distribution of genets in two species of Rhizopogon forming tuberculate ectomycorrhizas on Douglas fir. New Phytol 161:313–320
Kretzer AM, Dunham S, Molina R, Spatafora JW (2005) Patterns of vegetative growth and gene flow in Rhizopogon vinicolor and R-vesiculosus (Boletales, Basidiomycota). Mol Ecol 14:2259–2268
McCune B, Mefford MJ (2006) PC-ORD. multivariate analysis of ecological data, version 5. Gleneden Beach. MjM Software, OR
Miller SL, Koo CD, Molina R (1992) Early colonization of red alder and Douglas fir by ectomycorrhizal fungi and Frankia in soils from the Oregon coast range. Mycorrhiza 2:53–61
Ministry of Environment (2015) Red list of Japanese threatened species. http://ikilog.biodic.go.jp/Rdb/. Accessed July 2016
Miyamoto Y, Nara K (2016) Soil propagule banks of ectomycorrhizal fungi share many common species along an elevation gradient. Mycorrhiza 26:189–197
Mujic AB, Hosaka K, Spatafora JW (2014) Rhizopogon togasawariana sp nov., the first report of Rhizopogon associated with an Asian species of Pseudotsuga. Mycologia 106:105–112
Murata M, Kinoshita A, Nara K (2013) Revisiting the host effect on ectomycorrhizal fungal communities: implications from host-fungal associations in relict Pseudotsuga japonica forests. Mycorrhiza 23:641–653
Nara K (2006a) Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol 169:169–178
Nara K (2006b) Pioneer dwarf willow may facilitate tree succession by providing late colonizers with compatible ectomycorrhizal fungi in a primary successional volcanic desert. New Phytol 171:187–198
Nara K (2009) Spores of ectomycorrhizal fungi: ecological strategies for germination and dormancy. New Phytol 181:245–248
Nara K, Nakaya H, Wu BY, Zhou ZH, Hogetsu T (2003) Underground primary succession of ectomycorrhizal fungi in a volcanic desert on Mount Fuji. New Phytol 159:743–756
Nguyen NH, Hynson NA, Bruns TD (2012) Stayin’ alive: survival of mycorrhizal fungal propagules from 6-year-old forest soil. Fungal Ecol 5:741–746
Oksanen JBG, Kindt R, Legendre P, O’Hara B, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2010) Package ‘vegan’ community ecology package. R Package Version 1:17–2
Outerbridge RA, Trofymow JA, Lalumière A (2009) Re-establishment of ectomycorrhizae from refugia bordering regenerating Douglas-fir stands in Vancouver Island. Canadian Forest Service Publications, Victoria
Peay KG, Garbelotto M, Bruns TD (2009) Spore heat resistance plays an important role in disturbance-mediated assemblage shift of ectomycorrhizal fungi colonizing Pinus muricata seedlings. J Ecol 97:537–547
Pickles BJ, Gorzelak MA, Green DS, Egger KN, Massicotte HB (2015) Host and habitat filtering in seedling root-associated fungal communities: taxonomic and functional diversity are altered in ‘novel’ soils. Mycorrhiza 25:517–531
R Core Team (2014) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. http://www.R-project.org/
Rusca TA, Kennedy PG, Bruns TD (2006) The effect of different pine hosts on the sampling of Rhizopogon spore banks in five Eastern Sierra Nevada forests. New Phytol 170:551–560
Simard SW, Beiler KJ, Bingham MA, Deslippe JR, Philip LJ, Teste FP (2012) Mycorrhizal networks: mechanisms, ecology and modelling. Fungal Biol Rev 26:39–60
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn edn. Academic Press, London
Taylor DL, Bruns TD (1999) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Mol Ecol 8:1837–1850
Tedersoo L, Jairus T, Horton BM, Abarenkov K, Suvi T, Saar I, Koljalg U (2008) Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol 180:479–490
Twieg BD, Durall DM, Simard SW (2007) Ectomycorrhizal fungal succession in mixed temperate forests. New Phytol 176:437–447
Visser S (1995) Ectomycorrhizal fungal succession in jack pine stands following wildfire. New Phytol 129:389–401
Wei XX, Yang ZY, Li Y, Wang XQ (2010) Molecular phylogeny and biogeography of Pseudotsuga (Pinaceae): insights into the floristic relationship between Taiwan and its adjacent areas. Mol Phylogenet Evol 55:776–785
Wen ZG, Murata MS, Xu ZY, Chen YH, Nara K (2015) Ectomycorrhizal fungal communities on the endangered Chinese Douglas-fir (Pseudotsuga sinensis) indicating regional fungal sharing overrides host conservatism across geographical regions. Plant Soil 387:189–199
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: a guide to methods and applications. Academic Press, Berkeley
Yabe A (2011) Pseudotsuga tanaii Huzioka from the earliest Miocene Shichiku Flora of northeast Japan: systematics and ecological conditions. Paleontol Res 15:1–11
Yamanaka T (1975) Ecology of Pseudotsuga japonica and other coniferous forests in eastern Shikoku (in Japanese with English summary). Mem Natl Sci Mus 8:119–136
Yatoh K (1958) Materials for the botanical study on the forest flora of the Kii Peninsula. Analysis and classification of the forest communities (in Japanese). Bull Fac Agr Mie Univ 18:105–167
Acknowledgements
We thank members of the Aki District Forest Office and the Mie District Forest Office, and Mikio Mochizuki from the Kawakita Forest Office, for their assistance. This study was supported in part by a Grant for Japan-related Research Projects from The Sumitomo Foundation to KN and JSPS KAKENHI Grants to MM (24780143) and KN (21658054, 25660115, 15H02449).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Murata, M., Nagata, Y. & Nara, K. Soil spore banks of ectomycorrhizal fungi in endangered Japanese Douglas-fir forests. Ecol Res 32, 469–479 (2017). https://doi.org/10.1007/s11284-017-1456-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-017-1456-1