Abstract
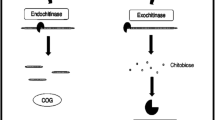
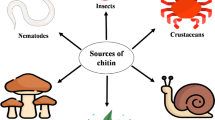
Chitinases are a group of hydrolytic enzymes that catalyze chitin, nd are synthesized by a wide variety of organisms. In nature, microbial chitinases are primarily responsible for chitin decomposition. Several chitinases have been reported and characterized, and they are garnering increasing attention for their uses in a wide range of applications. In the food industry, the direct fermentation of seafood, such as crab and shrimp shells, using chitinolytic microorganisms has contributed to increased nutritional benefits through the enhancement of chitin degradation into chitooligosaccharides. These compounds have been demonstrated to improve human health through their antitumor, antimicrobial, immunomodulatory, antioxidant, and anti-inflammatory properties. Moreover, chitinase and chitinous materials are used in the food industry for other purposes, such as the production of single-cell proteins, chitooligosaccharides, N-acetyl d-glucosamines, biocontrol, functional foods, and various medicines. The functional properties and hydrolyzed products of chitinase, however, depend upon its source and physicochemical characteristics. The present review strives to clarify these perspectives and critically discusses the advances and limitations of microbial chitinase in the further production of functional foods.


Similar content being viewed by others
References
Aam BB, Heggset EB, Norberg AL, Sørlie M, Vårum KM, Eijsink VG (2010) Production of chitooligosaccharides and their potential applications in medicine. Mar Drugs 8:1482–1517
Adrangi S, Faramarzi MA (2013) From bacteria to human: a journey into the world of chitinases. Biotechnol Adv 31:1786–1795
Ahsan SM, Thomas M, Reddy KK, Sooraparaju SG, Asthana A, Bhatnagar I (2018) Chitosan as biomaterial in drug delivery and tissue engineering. Int J Biol Macromol 110:97–109
Allonsius CN et al (2019) Inhibition of Candida albicans morphogenesis by chitinase from Lactobacillus rhamnosus GG. Sci Rep 9:2900
Alsina C, Faijes M, Planas A (2019) Glycosynthase-type GH18 mutant chitinases at the assisting catalytic residue for polymerization of chitooligosaccharides. Carbohydr Res 478:1–9
Barthomeuf C, Regerat F, Pourrat H (1994) Production, purification and characterization of a tannase from Aspergillus niger LCF 8. J Ferment Bioeng 77:320–323
Bekale L, Agudelo D, Tajmir-Riahi H (2015) Effect of polymer molecular weight on chitosan–protein interaction. Colloids Surf B 125:309–317
Belmares R, Contreras-Esquivel JC, Rodrı́guez-Herrera R, Coronel AR, Aguilar CN (2004) Microbial production of tannase: an enzyme with potential use in food industry. LWT-Food Sci Technol 37:857–864
Benhabiles M, Salah R, Lounici H, Drouiche N, Goosen M, Mameri N (2012) Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll 29:48–56
Berger LR, Reynold DM (1958) The chitinase system of a strain of Streptomyces griseus. Biochim Biophys Acta 29:522–534
Berini F, Katz C, Gruzdev N, Casartelli M, Tettamanti G, Marinelli F (2018) Microbial and viral chitinases: attractive biopesticides for integrated pest management. Biotechnol Adv 36:818–838
Bhatnagar A, Sillanpää M (2009) Applications of chitin-and chitosan-derivatives for the detoxification of water and wastewater—a short review. Adv Colloid Interface Sci 152:26–38
Brzezinska MS, Jankiewicz U, Burkowska A, Walczak M (2014) Chitinolytic microorganisms and their possible application in environmental protection. Curr Microbiol 68:71–81
Cardozo FA, Gonzalez JM, Feitosa VA, Pessoa A, Rivera ING (2017) Bioconversion of α-chitin into N-acetyl-glucosamine using chitinases produced by marine-derived Aeromonas caviae isolates. World J Microbiol Biotechnol 33:201
Castillo BM, Dunn MF, Navarro KG, Meléndez FH, Ortiz MH, Guevara SE, Palacios GH (2016) Antifungal performance of extracellular chitinases and culture supernatants of Streptomyces galilaeus CFFSUR-B12 against Mycosphaerella fijiensis Morelet. World J Microbiol Biotechnol 32:44
Chalutz E, Droby S (1998) Biological control of postharvest disease. In: Boland G (ed) Plant–microbe interactions and biological control. Dekker, New York, pp 157–170
Chang S-H, Lin Y-Y, Wu G-J, Huang C-H, Tsai GJ (2019) Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int J Biol Macromol 131:167–175
Consortium U (2018) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47:D506–D515
Cremar L, Gutierrez J, Martinez J, Materon L, Gilkerson R, Xu F, Lozano K (2018) Development of antimicrobial chitosan based nanofiber dressings for wound healing applications. Nanomed J 5:6–14
Dahiya N, Tewari R, Hoondal GS (2006) Biotechnological aspects of chitinolytic enzymes: a review. Appl Microbiol Biotechnol 71:773–782
Deng J-J, Shi D, Mao H-H, Li Z-W, Liang S, Ke Y, Luo X-C (2019) Heterologous expression and characterization of an antifungal chitinase (Chit46) from Trichoderma harzianum GIM 3.442 and its application in colloidal chitin conversion. Int J Biol Macromol. 134:113–121
Dev A et al (2010) Novel carboxymethyl chitin nanoparticles for cancer drug delivery applications. Carbohydr Polym 79:1073–1079
Duo-Chuan L (2006) Review of fungal chitinases. Mycopathologia 161:345–360
Dutta PK (2016) Chitin and chitosan for regenerative medicine. Springer, New Delhi
Elieh-Ali-Komi D, Hamblin MR (2016) Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv Res 4:411
Fernandes JC, Tavaria FK, Soares JC, Ramos ÓS, Monteiro MJ, Pintado ME, Malcata FX (2008) Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. Food Microbiol 25:922–928
Funkhouser JD, Aronson NN (2007) Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol 7:96
Gao L, Sun J, Secundo F, Gao X, Xue C, Mao X (2018) Cloning, characterization and substrate degradation mode of a novel chitinase from Streptomyces albolongus ATCC 27414. Food Chem 261:329–336
Giaever G et al (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387
Gortari MC, Hours RA (2008) Fungal chitinases and their biological role in the antagonism onto nematode eggs. A review. Mycol Prog 7:221–238
Gutiérrez-Román MI, Holguín-Meléndez F, Dunn MF, Guillén-Navarro K, Huerta-Palacios G (2015) Antifungal activity of Serratia marcescens CFFSUR-B2 purified chitinolytic enzymes and prodigiosin against Mycosphaerella fijiensis, causal agent of black Sigatoka in banana (Musa spp.). BioControl 60:565–572
Halbedel S et al (2019) A Listeria monocytogenes ST2 clone lacking chitinase ChiB from an outbreak of non-invasive gastroenteritis. Emerg Microbes Infect 8:17–28
Halder SK, Pal S, Mondal KC (2019) Biosynthesis of fungal chitinolytic enzymes and their potent biotechnological appliances. In: Yadav A, Mishra S, Singh S, Gupta A (eds) Recent advancement in white biotechnology through fungi. Springer, Cham, pp 281–298
Hamid R, Khan MA, Ahmad M, Ahmad MM, Abdin MZ, Musarrat J, Javed S (2013) Chitinases: an update. J Pharm Bioallied Sci 5:21
Han B et al (2016) Characterization of the first fungal glycosyl hydrolase family 19 Chitinase (NbchiA) from Nosema bombycis (Nb). J Eukaryot Microbiol 63:37–45
Hou F et al (2019) Activation and conformational changes of chitinase induced by ultrasound. Food Chem 285:355–362
Huang Q-S et al (2011) The GH18 family of chitinases: their domain architectures, functions and evolutions. Glycobiology 22:23–34
Hurtado-Guerrero R, van Aalten DM (2007) Structure of Saccharomyces cerevisiae chitinase 1 and screening-based discovery of potent inhibitors. Chem Biol 14:589–599
Itoh T et al (2013) Cooperative degradation of chitin by extracellular and cell surface-expressed chitinases from Paenibacillus sp. strain FPU-7. Appl Environ Microbiol 79:7482–7490
Itoh T, Kimoto H (2019) Bacterial chitinase system as a model of chitin biodegradation. In: Yang Q, Fukamizo T (eds) Targeting chitin-containing organisms. Springer, Singapore, pp 131–151
Jahromi ST, Barzkar N (2018) Marine bacterial chitinase as sources of energy, eco-friendly agent, and industrial biocatalyst. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2018.09.083
Jiang C et al (2014) Deoxycholic acid-modified chitooligosaccharide/mPEG-PDLLA mixed micelles loaded with paclitaxel for enhanced antitumor efficacy. Int J Pharm 475:60–68
Juárez-Hernández EO, Casados-Vázquez LE, Brieba LG, Torres-Larios A, Jimenez-Sandoval P, Barboza-Corona JE (2019) The crystal structure of the chitinase ChiA74 of Bacillus thuringiensis has a multidomain assembly. Sci Rep 9:2591
Jung W-J, Park R-D (2014) Bioproduction of chitooligosaccharides: present and perspectives. Mar Drugs 12:5328–5356
Junges  et al (2014) Genomic analyses and transcriptional profiles of the glycoside hydrolase family 18 genes of the entomopathogenic fungus Metarhizium anisopliae. PLoS ONE 9:e107864
Keyhani NO, Roseman S (1999) Physiological aspects of chitin catabolism in marine bacteria. Biochim Biophys Acta (BBA) Gen Subj 1473:108–122.
Kramer KJ, Muthukrishnan S (1997) Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem Mol Biol 27:887–900
Kumari S, Rath P, Kumar ASH, Tiwari T (2015) Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ Technol Innov 3:77–85
Kuranda MJ, Robbins PW (1991) Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J Biol Chem 266:19758–19767
Kusaoke H, Shinya S, Fukamizo T, Kimoto H (2017) Biochemical and biotechnological trends in chitin, chitosan, and related enzymes produced by Paenibacillus IK-5 Strain. Int J Biol Macromol 104:1633–1640
Langner T, Göhre V (2016) Fungal chitinases: function, regulation, and potential roles in plant/pathogen interactions. Curr Genet 62:243–254
Langner T, Öztürk M, Hartmann S, Cord-Landwehr S, Moerschbacher B, Walton JD, Göhre V (2015) Chitinases are essential for cell separation in Ustilago maydis. Eukaryot Cell 14:846–857
Le B, Yang SH (2018) Characterization of a chitinase from Salinivibrio sp. BAO-1801 as an antifungal activity and a biocatalyst for producing chitobiose. J Basic Microbiol 58:848–856
Lee H-W, Park Y-S, Jung J-S, Shin W-S (2002) Chitosan oligosaccharides, dp 2–8, have prebiotic effect on the Bifidobacterium bifidium and Lactobacillus sp. Anaerobe 8:319–324
Lee Y-S et al (2007) Cloning, purification, and characterization of chitinase from Bacillus sp. DAU101. Bioresour Technol 98:2734–2741
Lekha P, Lonsane B (1994) Comparative titres, location and properties of tannin acyl hydrolase produced by Aspergillus niger PKL 104 in solid-state, liquid surface an submerged fermentations. Process Biochem 29:497–503
Leoni C, Volpicella M, Dileo M, Gattulli BA, Ceci LR (2019) Chitinases as food allergens. Molecules 24:2087
Liang S, Sun Y, Dai X (2018) A review of the preparation, analysis and biological functions of chitooligosaccharide. Int J Mol Sci 19:2197
Liaqat F, Eltem R (2018) Chitooligosaccharides and their biological activities: a comprehensive review. Carbohydr Polym 184:243–259
Liu J, NanGong Z, Zhang J, Song P, Tang Y, Gao Y, Wang Q (2019) Expression and characterization of two chitinases with synergistic effect and antifungal activity from Xenorhabdus nematophila. World J Microbiol Biotechnol 35:106
Madhuprakash J, Dalhus B, Vaaje-Kolstad G, Sakuda S, Podile AR, Eijsink VG, Sørlie M (2019) Structural and thermodynamic signatures of ligand binding to the enigmatic chitinase D of Serratia proteamaculans. J Phys Chem B 123:2270–2279
Mattaveewong T, Wongkrasant P, Chanchai S, Pichyangkura R, Chatsudthipong V, Muanprasat C (2016) Chitosan oligosaccharide suppresses tumor progression in a mouse model of colitis-associated colorectal cancer through AMPK activation and suppression of NF-κB and mTOR signaling. Carbohydr Polym 145:30–36
Meibom KL, Li XB, Nielsen AT, Wu C-Y, Roseman S, Schoolnik GK (2004) The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci 101:2524–2529
Miksusanti M, Saputra H, Sandi S, Hermansyah H (2016) The effect of Lactobacillus acidophilus and chito-oligosaccharide on antibacterial activity and organic acid production. IJFAC (Indones J Fundam Appl Chem) 1:29–34
Monreal J, Reese ET (1969) The chitinase of Serratia marcescens. Can J Microbiol 15:689–696
Murao S, Kawada T, Itoh H, Oyama H, Shin T (1992) Purification and characterization of a novel type of chitinase from Vibrio alginolyticus TK-22. Biosci Biotechnol Biochem 56:368–369
Muzzarelli RA (2013) Chitin. Elsevier, Kent
Mythili J, Chethana B, Rajeev P, Ganeshan G (2018) Chitinase gene construct from Trichoderma harzianum proved effective against onion purple blotch caused by Alternaria porri
Nasseri A, Rasoul-Amini S, Morowvat M, Ghasemi Y (2011) Single cell protein: production and process. Am J Food Technol 6:103–116
Ohishi K, Murase K, Ohta T, Etoh H (2000) Cloning and sequencing of a chitinase gene from Vibrio alginolyticus H-8. J Biosci Bioeng 89:501–505
Pan M, Li J, Lv X, Du G, Liu L (2019) Molecular engineering of chitinase from Bacillus sp. DAU101 for enzymatic production of chitooligosaccharides. Enzyme Microb Technol 124:54–62
Pan XQ, Shih CC, Harday J (2005) Chitinase induces lysis of MCF-7 cells in culture and of human breast cancer xenograft B11–2 in SCID mice. Anticancer Res 25:3167–3172
Park JK, Chung MJ, Choi HN, Park YI (2011) Effects of the molecular weight and the degree of deacetylation of chitosan oligosaccharides on antitumor activity. Int J Mol Sci 12:266–277
Patel S, Goyal A (2011) Functional oligosaccharides: production, properties and applications. World J Microbiol Biotechnol 27:1119–1128
Patil NS, Jadhav JP (2014) Enzymatic production of N-acetyl-D-glucosamine by solid state fermentation of chitinase by Penicillium ochrochloron MTCC 517 using agricultural residues. Int Biodeterior Biodegrad 91:9–17
Paulsen SS, Strube ML, Bech PK, Gram L, Sonnenschein EC (2019) Marine chitinolytic Pseudoalteromonas represents an untapped reservoir of bioactive potential. mSystems. https://doi.org/10.1128/mSystems.00060-19
Pinnamaneni R, Kalidas P, Rao KS (2010) Cloning and expression of Bbchit1 gene of Beauveria bassiana. J Entomol 4:30–35
Rathore AS, Gupta RD (2015) Chitinases from bacteria to human: properties, applications, and future perspectives. Enzyme Res. https://doi.org/10.1155/2015/791907
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Robbins PW, Albright C, Benfield B (1988) Cloning and expression of a Streptomyces plicatus chitinase (chitinase-63) in Escherichia coli. J Biol Chem 263:443–447
Rødde RH, Einbu A, Vårum KM (2008) A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalus borealis). Carbohydr Polym 71:388–393
Sahai A, Manocha M (1993) Chitinases of fungi and plants: their involvement in morphogenesis and host–parasite interaction. FEMS Microbiol Rev 11:317–338
Samain E, Drouillard S, Heyraud A, Driguez H, Geremia RA (1997) Gram-scale synthesis of recombinant chitooligosaccharides in Escherichia coli. Carbohydr Res 302:35–42
Selenius O, Korpela J, Salminen S, Gallego CG (2018) Effect of chitin and chitooligosaccharide on in vitro growth of Lactobacillus rhamnosus GG and Escherichia coli TG. Appl Food Biotechnol 5:163–172
Sharma R, Singh D, Singh R (2009) Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol Control 50:205–221
Sousa AJ et al (2019) A thermostable chitinase from the antagonistic Chromobacterium violaceum that inhibits the development of phytopathogenic fungi. Enzyme Microb Technol 126:50–61
Stoykov YM, Pavlov AI, Krastanov AI (2015) Chitinase biotechnology: production, purification, and application. Eng Life Sci 15:30–38
Sun X, Li Y, Tian Z, Qian Y, Zhang H, Wang L (2019) A novel thermostable chitinolytic machinery of Streptomyces sp. F-3 consisting of chitinases with different action modes. Biotechnol Biofuels 12:136
Suresh P, Kumar PA (2012) Enhanced degradation of α-chitin materials prepared from shrimp processing byproduct and production of N-acetyl-D-glucosamine by thermoactive chitinases from soil mesophilic fungi. Biodegradation 23:597–607
Suzuki K, Mikami T, Okawa Y, Tokoro A, Suzuki S, Suzuki M (1986) Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose. Carbohydr Res 151:403–408
Take K, Fujiki H, Suyotha W, Hayashi J, Takagi K, Yano S, Wakayama M (2018) Enzymatic and molecular characterization of an acidic and thermostable chitinase 1 from Streptomyces thermodiastaticus HF 3–3. J Gen Appl Microbiol. https://doi.org/10.2323/jgam.2017.12.002
Tanaka H, Phaff HJ (1965) Enzymatic hydrolysis of yeast cell walls I. Isolation of wall-decomposing organisms and separation and purification of lytic enzymes. J Bacteriol 89:1570–1580
Tanaka T, Fukui T, Atomi H, Imanaka T (2003) Characterization of an exo-β-D-glucosaminidase involved in a novel chitinolytic pathway from the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J Bacteriol 185:5175–5181
Terrapon N, Lombard V, Drula E, Coutinho PM, Henrissat B (2017) The CAZy database/the carbohydrate-active enzyme (CAZy) database: principles and usage guidelines. In: Aoki-Kinoshita K (ed) A practical guide to using glycomics databases. Springer, Tokyo, pp 117–131
Thanou M, Florea B, Geldof M, Junginger H, Borchard G (2002) Quaternized chitosan oligomers as novel gene delivery vectors in epithelial cell lines. Biomaterials 23:153–159
Tom RA, Carroad PA (1981) Effect of reaction conditions on hydrolysis of chitin by Serratia marcescens QMB 1466 chitinase. J Food Sci 46:646–647
Tuveng TR, Hagen LH, Mekasha S, Frank J, Arntzen MØ, Vaaje-Kolstad G, Eijsink VG (2017) Genomic, proteomic and biochemical analysis of the chitinolytic machinery of Serratia marcescens BJL200. Biochim Biophys Acta (BBA)—Proteins Proteom 1865:414–421
Vaaje-Kolstad G, Horn SJ, Sørlie M, Eijsink VG (2013) The chitinolytic machinery of Serratia marcescens—a model system for enzymatic degradation of recalcitrant polysaccharides. FEBS J 280:3028–3049
Vaikuntapu PR, Rambabu S, Madhuprakash J, Podile AR (2016) A new chitinase-D from a plant growth promoting Serratia marcescens GPS5 for enzymatic conversion of chitin. Bioresour Technol 220:200–207
Vyas P, Deshpande M (1991) Enzymatic hydrolysis of chitin by Myrothecium verbucaria chitinase complex and its utilization to produce SCP. J Gen Appl Microbiol 37:267–275
Wadhwa M, Bakshi M (2016) Application of waste-derived proteins in the animal feed industry. In: Protein byproducts. Elsevier, pp 161–192
Watanabe T, Kanai R, Kawase T, Tanabe T, Mitsutomi M, Sakuda S, Miyashita K (1999) Family 19 chitinases of Streptomyces species: characterization and distribution. Microbiology 145:3353–3363
Watanabe T, Kobori K, Miyashita K, Fujii T, Sakai H, Uchida M, Tanaka H (1993) Identification of glutamic acid 204 and aspartic acid 200 in chitinase A1 of Bacillus circulans WL-12 as essential residues for chitinase activity. J Biol Chem 268:18567–18572
Watanabe T, Oyanagi W, Suzuki K, Ohnishi K, Tanaka H (1992) Structure of the gene encoding chitinase D of Bacillus circulans WL-12 and possible homology of the enzyme to other prokaryotic chitinases and class III plant chitinases. J Bacteriol 174:408–414
Yandigeri MS, Malviya N, Solanki MK, Shrivastava P, Sivakumar G (2015) Chitinolytic Streptomyces vinaceusdrappus S5MW2 isolated from Chilika lake, India enhances plant growth and biocontrol efficacy through chitin supplementation against Rhizoctonia solani. World J Microbiol Biotechnol 31:1217–1225
Zhang A, He Y, Wei G, Zhou J, Dong W, Chen K, Ouyang P (2018) Molecular characterization of a novel chitinase Cm Chi1 from Chitinolyticbacter meiyuanensis SYBC-H1 and its use in N-acetyl-d-glucosamine production. Biotechnol Biofuels 11:179
Zhou J, Chen L, Kang L, Liu Z, Bai Y, Yang Y, Yuan S (2018) ChiE1 from Coprinopsis cinerea is characterized as a processive exochitinase and revealed to have a significant synergistic action with endochitinase ChiIII on chitin degradation. J Agric Food Chem 66:12773–12782
Zou P et al (2019) Structural characterization and antitumor effects of chitosan oligosaccharides against orthotopic liver tumor via NF-κB signaling pathway. J Funct Foods 57:157–165
Zou P, Yuan S, Yang X, Zhai X, Wang J (2018) Chitosan oligosaccharides with degree of polymerization 2–6 induces apoptosis in human colon carcinoma HCT116 cells. Chem Biol Interact 279:129–135
Acknowledgements
This study was carried out with the support of the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. NRF-2017R1D1A3B03027816).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Le, B., Yang, S.H. Microbial chitinases: properties, current state and biotechnological applications. World J Microbiol Biotechnol 35, 144 (2019). https://doi.org/10.1007/s11274-019-2721-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-019-2721-y