Abstract
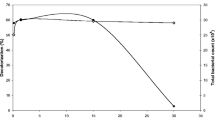
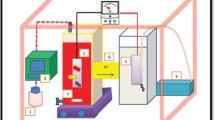
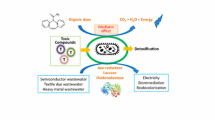
Dissimilatory metal reducing bacteria can exchange electrons extracellularly and hold great promise for their use in simultaneous wastewater treatment and electricity production. This study investigated the role of riboflavin, an electron carrier, in the decolourisation of Congo red in microbial fuel cells (MFCs) using Shewanella oneidensis MR-1 as a model organism. The contribution of the membrane-bound protein MtrC to the decolourisation process was also investigated. Within the range of riboflavin concentrations tested, 20 µM was found to be the best with >95% of the dye (initial concentration 200 mg/L) decolourised in MFCs within 50 h compared to 90% in the case where no riboflavin was added. The corresponding maximum power density was 45 mW/m2. There was no significant difference in the overall decolourisation efficiencies of Shewanela oneidensis MR-1 ΔMtrC mutants compared to the wild type. However, in terms of power production the mutant produced more power (Pmax 76 mW/m2) compared to the wild type (Pmax 46 mW/m2) which was attributed to higher levels of riboflavin secreted in solution. Decolourisation efficiencies in non-MFC systems (anaerobic bottles) were similar to those under MFC systems indicating that electricity generation in MFCs does not impair dye decolourisation efficiencies. The results suggest that riboflavin enhances both decolourisation of dyes and simultaneous electricity production in MFCs.








Similar content being viewed by others
References
Carmona-Martinez AA, Harnisch F, Fitzgerald LA, Biffinger JC, Ringeisen BR, Schroder U (2011) Cyclic voltammetric analysis of the electron transfer of Shewanella oneidensis MR-1 and nanofilament and cytochrome knock-out mutants. Bioelectrochemistry 81:74–80
Chen BY, Wang YM, Ng IS (2011) Understanding interactive characteristics of bioelectricity generation and reductive decolourisation using Proteus hauseri. Bioresour Technol 102:1159–1165
Chen BY, Hong J, Ng IS, Wang YM, Liu SQ, Lin B, Ni C (2013) Deciphering simultaneous bioelectricity generation and reductive decolourisation using mixed-culture microbial fuel cells in salty media. J Taiwan Inst Chem Eng 44:446–453
Edwards MJ, White GF, Norman M, Fernandez AT, Ainsworth E, Shi L, Fredrickson JK, Zachara JM, Butt JN, Richardson DJ, Clarke TA (2015) Redox linked flavin sites in extracellular decaheme proteins involved in microbe-mineral electron transfer. Sci Rep 5:11677. doi:10.1038/srep11677
Fernando E (2014). Treatment of azo dyes in industrial wastewater using microbial fuel cells. PhD thesis, University of Westminster, London, UK
Hong Y, Chen X, Guo J, Xu Z, Xu M, Sun G (2007) Effects of electron donors and acceptors on anaerobic reduction of azo dyes by Shewanella decolorationis S12. Appl Microbiol Biotechnol 74:230–238
Hou B, Sun J, Hu S (2011) Effect of enrichment procedures on performance and microbial diversity of microbial fuel cell for Congo red decolourisation and electricity generation. Appl Microbiol Biotechnol 90:1563–1572
Hsueh CC, Wang YM, Chen BY (2014) Metabolite analysis on reductive biodegradation of reactive green 19 in Enterobacter cancerogenus bearing microbial fuel cell (MFC) and non-MFC cultures. J Taiwan Inst Chem Eng 45:436–443
Imran M, Arshad M, Negm F, Khalid A, Shaharoona B, Hussain S, Nadeem SM, Crowley DE (2016) Yeast Extract promotes decolourisation of azo dyes by stimulating azoreductase activity in Shewanella sp strain IFN4. Ecotoxicol Environ Saf 124:42–49
Kotloski N.J, Gralnick J.A. (2013). Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis. mBio 4(1):e00553–12.
Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR (2008) Shewanella secretes flavins that mediate extracellular electron transfer. PNAS 105:7698–7973
Mohammadi A, Khalili B, Tahavor M (2015) Novel push–pull heterocyclic azo disperse dyes containing piperazine moiety: Synthesis, spectral properties, antioxidant activity and dyeing performance on polyester fibres. Spectrochim Acta Part A 150:799–805
Pandey P, Shinde V, Deopurkar JL, Pant D (2016) Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl Energy 168:706–723
Rau J, Knackmuss H-J, Stolz A (2002) Effect of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ Sci Technol 36:1497–1502
Ross DE, Brantley SL, Tien M (2009) Kinetic characterization of OmcA and MtrC, terminal reductases involved in respiratory electron transfer for dissimilatory iron reduction in Shewanella oneidensis MR-1. Appl Environ Microbiol 75:5218–5226
Saratale RG, Saratale GD, Chang JS, Govindar SP (2011) Bacterial decolourisation and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42(1):138–147
Sivashankar R, Sathya AB, Krishnakumar U, Sivasubramanian V (2015) Synthesis of magnetic biocomposite for efficient adsorption of azo dye from aqueous solution. Ecotoxicol Environ Saf 121:149–153
Solanki K, Subramanian S, Basu S (2013) Microbial fuel cells for azo dye treatment with electricity generation: a review. Bioresour Technol 131:564–571
Velasquez-Orta SB, Head IM (2010) The effect of flavin electron shuttles in microbial fuel cells current production. Appl Microbiol Biotechnol 85:1373–1381
Von Canstein H, Ogawa J, Shimizu S, Lloyd JR (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol 74:615–623
Watanabe K, Manefield M, Lee M, Kouzuma A (2009) Electron shuttles in biotechnology. Curr Opin Biotechnol 20:633–641
Xu Z, Lin Z, Wang Z, Chen T (2015) Improvement of the riboflavin production by engineering the precursor biosynthesis pathways in Escherichia coli. Chin J Chem Eng 23:1834–1839
Yong YC, Cai Z, Yu YY, Chen P, Jian R, Cao B, Sun JZ, Wang JY, Song H (2013) Increase of riboflavin biosynthesis underlies enhancement of extracellular electron transfer of Shewanella in alkaline microbial fuel cells. Bioresour Technol 130:763–768
Acknowledgements
The authors would like to thank the British Council for facilitating the travel of the first author to conduct this work at the department of Life Sciences at University of Westminster, London, UK through the travel grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomaa, O.M., Fapetu, S., Kyazze, G. et al. The role of riboflavin in decolourisation of Congo red and bioelectricity production using Shewanella oneidensis-MR1 under MFC and non-MFC conditions. World J Microbiol Biotechnol 33, 56 (2017). https://doi.org/10.1007/s11274-017-2223-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-017-2223-8