Abstract
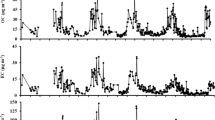
To investigate the characteristic distributions and formation mechanism of the polar organic components in atmospheric aerosols, atmospheric particulates were simultaneously sampled at three typical sites in urban(U), suburban(S) and rural(R) of Xi’an in summer and winter. Organic compositions, including dicarboxylic acids (DCAs), monocarboxylic acids (MCAs) were emphatically analyzed to explore the seasonal, spatial, and other variations. The results showed that total concentrations of DCAs and MCAs in winter were higher than those in summer. Athough the total content of DCAs in PM10 in winter is lower than that of MCAs, the total concentration of DCAs in PM2.5 and PM10 in summer are higher than that of MCAs. The total concentration of DCAs in PM2.5 in winter and summer were 1878 ± 1425 ng/m3 and 1334 ± 493 ng/m3, 1294 ± 943 ng/m3 and 728 ± 477 ng/m3 in PM10. While the total concentration of MCAs in PM2.5 in winter and summer were 1193 ± 1142 ng/m3 and 541 ± 367 ng/m3, 1659 ± 1162 ng/m3 and 492 ± 424 ng/m3 in PM10. Urban subtotal concentrations of DCAs from PM2.5 were the highest, and mass of organic acids in sunny days were significantly lower than the hazy days. Strong correlations were observed among the DCAs with ambient oxidants and precursors. Moreover, DCAs concentrations were vulnerable to the environmental factors, such as light intensity and relative humidity. Comparisons demonstrated that MCAs mainly comes from coal combustion, vehicle exhaust and other primary emissions, while the secondary photochemistry and liquid phase oxidation are the main sources of DCAs.





Similar content being viewed by others
References
Bikkina, S., Kawamura, K., & Sarin, M. (2017). Secondary organic aerosol formation over Coastal Ocean: Inferences from atmospheric water-soluble low molecular weight organic compounds. Environmental Science & Technology, 51, 4347–4357. https://doi.org/10.1021/acs.est.6b05986.
Cruz, L. P. S., Mota, E. R., Campos,V. P., Santana, F. O., Luz,S. R., & Santos. D. F. (2019). Inorganic and Organic Acids in the Atmosphere of the Urban Area of the City of Salvador, Brazil. Journal of the Brazilian Chemical Society, 30, 904–914. https://doi.org/10.21577/0103-5053.20180227.
Carlton, A. G., Turpin, B. J., Altieri, K. E., Seitzinger, S., Reff, A., Lim, H. J., & Ervens, B. (2007). Atmospheric oxalic acid and SOA production from glyoxal: Results of aqueous photooxidation experiments. Atmospheric Environment, 41, 7588–7602. https://doi.org/10.1016/j.atmosenv.2007.05.035.
Chattopadhyay, S., & Ziemann, P. J. (2005). Vapor pressures of substituted and unsubstituted monocarboxylic and dicarboxylic acids measured using an improved thermal desorption particle beam mass spectrometry method. Aerosol Science and Technology, 39, 1085–1100. https://doi.org/10.1080/02786820500421547.
Du, W., Hong, Z., Chen, Y., Deng, J., Chen, J., Xu, L., & Xiao, H. (2017). Spatiotemporal distribution and source apportionment of low molecular weight organic acids in wet precipitation at a coastal city, China. Environmetal Science and Pollution Research International, 24, 8399–8410. https://doi.org/10.1007/s11356-017-8498-3.
Deshmukh, D. K., Kawamura, K., & Deb, M. K. (2016). Dicarboxylic acids, ω-oxocarboxylic acids, α-dicarbonyls, WSOC, OC, EC, and inorganic ions in wintertime size-segregated aerosols from Central India: Sources and formation processes. Chemosphere, 161, 27–42. https://doi.org/10.1016/j.chemosphere.2016.06.107.
Gowda, D., & Kawamura, K. (2018). Seasonal variations of low molecular weight hydroxy-dicarboxylic acids and oxaloacetic acid in remote marine aerosols from Chichijima Island in the western North Pacific (December 2010–November 2011). Atmospheric Research, 204, 128–135. https://doi.org/10.1016/j.atmosres.2018.01.007.
Guo, H., Zhou, J., Wang, L., Zhou, Y., Yuan, J., & Zhao, R. (2015). Seasonal variations and sources of carboxylic acids in PM2. 5 in Wuhan, China. Aerosol air Qual. Res, 15, 517–528. https://doi.org/10.4209/aaqr.2014.02.0040.
He, L. Y., Hu, M., Huang, X. F., Zhang, Y., & Tang, X. (2006). Seasonal pollution characteristics of organic compounds in atmospheric fine particles in Beijing. Science of the Total Environment, 359, 167–176. https://doi.org/10.1016/j.scitotenv.2005.05.044.
Hoque, M. M., & Kawamura, K. (2016). Longitudinal distributions of dicarboxylic acids, ω-oxoacids, pyruvic acid, α-dicarbonyls, and fatty acids in the marine aerosols from the Central Pacific including equatorial upwelling. Global Biogeochemical Cycles, 30, 534–548. https://doi.org/10.1002/2015GB005346.
Ho, K. F., Lee, S. C., Ho, S. S., Kawamura, K., Tachibana, E., Cheng, Y., & Zhu, T. (2010). Dicarboxylic acids, ketocarboxylic acids, α-dicarbonyls, fatty acids, and benzoic acid in urban aerosols collected during the 2006 campaign of air quality research in Beijing (CAREBeijing-2006). Journal of Geophysical Research-Atmospheres, 115(D19). https://doi.org/10.1029/2009JD013304.
Hellén, H., Schallhart, S., Praplan, A. P., Petäjä, T., & Hakola, H. (2017). Using in situ GC-MS for analysis of C2-C7 volatile organic acids in ambient air of a boreal forest site. Atmospheric Measurement Techniques, 10, 281. https://doi.org/10.5194/amt-10-281-2017.
Hou, X., Zhuang, G., Sun, Y., & An, Z. (2006). Characteristics and sources of polycyclic aromatic hydrocarbons and fatty acids in PM2.5 aerosols in dust season in China. Atmospheric Environment, 40, 3251–3326. https://doi.org/10.1016/j.atmosenv.2006.02.003.
Kawamura, K., & Bikkina, S. (2016). A review of dicarboxylic acids and related compounds in atmospheric aerosols: Molecular distributions, sources and transformation. Atmospheric Research, 170, 140–160. https://doi.org/10.1016/j.atmosres.2015.11.018.
Kim, H., Collier, S., Ge, X., Xu, J., Sun, Y., Jiang, W., Wang, Y., Herckes, P., & Zhang, Q. (2019). Chemical processing of water-soluble species and formation of secondary organic aerosol in fogs. Atmospheric Environment, 200, 158–166. https://doi.org/10.1016/j.atmosenv.2018.11.062.
Kunwar, B., Kawamura, K., Fujiwara, S., Fu, P., Miyazaki, Y., & Pokhrel, A. (2019). Dicarboxylic acids, oxocarboxylic acids and α-dicarbonyls in atmospheric aerosols from Mt. Fuji, Japan: Implication for primary emission versus secondary formation. Atmospheric Research, 221, 58–71. https://doi.org/10.1016/j.atmosres.2019.01.021.
Kawamura, K., & Yasui, O. (2005). Diurnal changes in the distribution of dicarboxylic acids, ketocarboxylic acids and dicarbonyls in the urban Tokyo atmosphere. Atmospheric Environment, 39, 1945–1960. https://doi.org/10.1016/j.atmosenv.2004.12.014.
Lange, R., Dall'Osto, M., Wex, H., Skov, H., & Massling, A. (2019). Large summer contribution of organic biogenic aerosols to Arctic cloud condensation nuclei. Geophysical Research Letters, 46, 11500–11509. https://doi.org/10.1029/2019GL084142.
Mochizuki, T., Kawamura, K., Aoki, K., & Sugimoto, N. (2016). Long-range atmospheric transport of volatile monocarboxylic acids with Asian dust over a high mountain snow site, Central Japan. Atmospheric Chemistry and Physics, 16, 14621–14633. https://doi.org/10.5194/acp-16-14621-2016.
Martinez, R. E., Williams, B. J., Zhang,Y., Hangan, D., Walker, M., Kreisberg, N. M., Hering, S. V., Hohaus, T., Jayne, J. T., & Warsnop, D. R. (2016). Development of a volatility and polarity separator (VAPS) for volatility-and polarity-resolved organic aerosol measurement. Aerosol Science and Technology, 50, 255–271. https://doi.org/10.1080/02786826.2016.1147645.
McVay, R. C., Zhang, X., Aumont, B., Valorso, R., Camredon, M., La, Y. S., & Seinfeld, J. H. (2016). SOA formation from the photooxidation of α-pinene: Systematic exploration of the simulation of chamber data. Atmospheric Chemistry and Physics, 16, 2785–2802. https://doi.org/10.5194/acp-16-2785-2016.
Peng, C., Jing, B., Guo, Y. C., Zhang, Y. H., & Ge, M. F (2016). Hygroscopic behavior of multicomponent aerosols involving NaCl and dicarboxylic acids. the Jounal of Physical Chemistry A, 120, 1029–1038. https://doi.org/10.1021/acs.jpca.5b09373
Pavuluri, C. M., Kawamura., K., Mihalopoulos, N., & Swaminathan, T. (2015). Laboratory photochemical processing of aqueous aerosols: Formation and degradation of dicarboxylic acids, oxocarboxylic acids and α-dicarbonyls. Atmospheric Chemistry and Physics, 15, 7999–8012. https://doi.org/10.5194/acp-15-7999-2015.
Posner, L. N., Theodoritsi, G., Robinson, A., Yarwood, G., Koo, B., Morris, R., Mavko, M., Moore, T., & Pandis, S. N. (2019). Simulation of fresh and chemically-aged biomass burning organic aerosol. Atmospheric Environment, 196, 27–37. https://doi.org/10.1016/j.atmosenv.2018.09.055.
Reyes-Villegas, E., Green, D. C., Priestman, M., Canonaco, F., Coe, H., Prevot, A. S. H., & Allan, D. J. (2016). Organic aerosol source apportionment in London 2013 with ME-2: Exploring the solution space with annual and seasonal analysis. Atmospheric Chemistry and Physics, 16, 15545–15559. https://doi.org/10.5194/acp-16-15545-2016.
Rogge, W. F., Hildemann, L. M., Mazurek, M. A., Cass, G. R., & Simoneit, B. R. (1998). Sources of fine organic aerosol. 9. Pine, oak, and synthetic log combustion in residential fireplaces. Environmental Science & Technology, 32, 13–22. https://doi.org/10.1021/es960930b.
Ren, Y., Li,H., Meng, F., Wang, G., Zhang, H., Yang, T., Li, W., Ji, Y., Bi, F., & Wang, X. (2019). Impact of emission controls on air quality in Beijing during the 2015 China victory Day parade: Implication from organic aerosols. Atmospheric Environment, 198, 207–214. https://doi.org/10.1016/j.atmosenv.2018.10.061.
Simoneit, B. R. T., Kobayashi, M., Mochida, M., Kawamura, K., & Huebert, B. J. (2004). Aerosol particles collected on aircraft flights over the northwestern Pacific region during the ACE-Asia campaign: Composition and major sources of the organic compounds. Journal of Geophysical Research: Atmospheres, 109, 1–15. https://doi.org/10.1029/2004JD004565.
Tedetti, M., Kawamura, K., Narukawa, M., Joux, F., Charrière, B., & Sempéré, R. (2007). Hydroxyl radical-induced photochemical formation of dicarboxylic acids from unsaturated fatty acid (oleic acid) in aqueous solution. Journal of Photochemistry and Photobiology A-Chemistry, 188, 135–139. https://doi.org/10.1016/j.jphotochem.2006.11.029.
Tong, H., Kourtchev, I., Pant, P., Keyte,I. J., Oconnor, I. P., Wenger,J. C., Pope, F. D., Horrison, R. M., & Kalberer. M. (2016). Molecular composition of organic aerosols at urban background and road tunnel sites using ultra-high resolution mass spectrometry. Faraday Discussions, 189, 51–68. https://doi.org/10.1039/C5FD00206K.
Thompson, S. L., Yatavelli, R. L., Stark, H., Kimmel, J. R., Krechmer, J. E., Day, D. A., & Khan, M. A. H. (2017). Field intercomparison of the gas/particle partitioning of oxygenated organics during the southern oxidant and aerosol study (SOAS) in 2013. Aerosol Science and Technology, 51, 30–56. https://doi.org/10.1080/02786826.2016.1254719.
Yu, Q., Chen, J., Qin, W., Cheng, S., Zhang, Y., Ahmad, M., & Ouyang, W. (2019). Characteristics and secondary formation of water-soluble organic acids in PM1, PM2. 5 and PM10 in Beijing during haze episodes. Science of the Total Environment, 669, 175–184. https://doi.org/10.1016/j.scitotenv.2019.03.131.
Yu, L. E., ShulmanM, L., Kopperud, R., & Hildemann, L. M. (2005). Characterization of organic compounds collected during southeastern aerosol and visibility study: Water-soluble organic species. Environmental Science & Technology, 39, 707–715. https://doi.org/10.1021/es0489700.
Zhang, Y. L., Cao, K., Kawamura, F., & Lee, M. (2016). Stable carbon isotopic compositions of low-molecular-weight dicarboxylic acids, oxocarboxylic acids, α-dicarbonyls, and fatty acids: Implications for atmospheric processing of organic aerosols. Journal of Geophysical Research: Atmospheres, 121, 3707–3717. https://doi.org/10.1029/2005JD006466.
Acknowledgements
The authors are thankful to the support of National Natural Science Foundation of China (No.21677115), and Shaanxi Provincial Key Research and Development Program (No.2019SF-238).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, L., Wei, L., Wang, G. et al. Comparison of Atmospheric Monocarboxylic and Dicarboxylic Acids in Xi’ an, China, for Source Apportionment of Organic Aerosols. Water Air Soil Pollut 231, 337 (2020). https://doi.org/10.1007/s11270-020-04675-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04675-y