Abstract
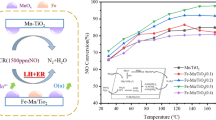
Micro-physicochemical characteristics and low-temperature SCR activities of the Mn–Ce–Cr catalysts on different carriers were investigated with SEM, XRD, XPS, FTIR, and denitration experiments. Mn–Ce–Cr catalysts carried on TiO2 and ZrO2, and composite carrier containing Al2O3 and TiO2 had visible element interactions on the surfaces, and Mn presented the mixed valences of Mn3+ and Mn4+. Mn3+ was transferred to Mn4+ due to the oxidation processes of Ce3+ to Ce4+ and Cr3+ to Cr6+, and the Oα/Oβ ratio decreased during the SCR process. Compared with single carriers such as TiO2 or ZrO2, the catalysts on the composite carriers of Al2O3 and TiO2 had better pore structures and higher fractions of Mn4+, Ce3+, Cr6+, and chemisorbed oxygen. It could also absorb the coordination-state NH3 well, especially the higher activity l-acid sites during the SCR process, and contribute to the formation of composite oxide MnxTi1−xO on Mn–Ce–Cr/Al2O3+TiO2 catalyst. All the above factors had positive effects on the low-temperature SCR. However, Co-doping in Mn–Ce–Cr catalysts could not improve the pore structures or promote the dispersions of Mn–Ce–Cr active substances on the carrier surface. Groups such as nitrates and nitrites produced by NO adsorption would hinder the adsorption of NH3 and low-temperature SCR. Mn–Ce–Cr/Al2O3+TiO2 catalyst had high low-temperature SCR activity, while Mn–Ce–Cr/ZrO2 catalyst was the most unstable with the lowest denitration efficiency. Moreover, for the scrapped catalyst from coal-fired power plants, it could still be used as the carrier of the Mn–Ce–Cr catalyst, and its SCR characteristics were much better than the above catalysts, especially at broader temperature range.











Similar content being viewed by others
References
Asadullah, M., Asaduzzaman, M., Kabir, M. S., Mostofa, M. G., & Miyazawa, T. (2010). Chemical and structural evalution of activated carbon prepared from jute sticks for brilliant Green dye removal from aqueous solution. Journal of Hazardous Materials, 174, 437–443.
Centeno, M. A., Carrizosa, .I, & Odriozola, J. A. (2001). NO-NH3 coadsorption on vanadia/ titania catalysts: determination of the reduction degree of vanadium. Applied Catalysis B: Environmental, 29, 307–314. https://doi.org/10.1016/S0926-3373(00)00214-9.
Chen, L., Li, J., Ge, M., & Zhu, R. (2010a). Enhanced activity of tungsten modified CeO2/TiO2 for selective catalytic reduction of NOx with ammonia. Catalysis Today, 153, 77–83. https://doi.org/10.1016/j.cattod.2010.01.062.
Chen, Z. H., Yang, Q., Li, H., Li, X. H., Wang, L. F., & Tsang, S. C. (2010b). Cr-MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature. Journal of Catalysis, 276, 56–65. https://doi.org/10.1016/j.jcat.2010.08.016.
Chen, Q. L., Guo, R. T., Wang, Q. S., Pan, W. G., Wang, W. H., Yang, N. Z., Lu, C. Z, & Wang, S. X. (2016). The catalytic performance of Mn/TiWOx catalyst for selective catalytic reduction of NOx with NH3. Fuel, 181, 852–858. https://doi.org/10.1016/j.fuel.2016.05.045.
Chen, X. L., Ceng, Y., Shan, W. P., & Liu, F. D. (2018). Deactivation effects of potassium on a CeMoTiOx catalyst for the selective catalytic reduction of NOx with NH3. Industrial & Engineering Chemistry Research, 57, 1399–1407. https://doi.org/10.1021/acs.iecr.7b04444.
Costello, C. K., Yang, J. H., Law, H. Y., Wang, Y., Lin, J. N., Marks, L. D., Kung, M. C., & Kung, H. H. (2003). On the potential role of hydroxyl groups in CO oxidation over Au/Al2O3. Applied Catalysis A: General, 243, 15–24. https://doi.org/10.1016/S0926-860X(02)00533-1.
Craciun, R., Nentwick, B., Hadjiivanov, K., & Knozinger, H. (2003). Structure and redox properties of MnOx/yttrium-stabilized-zirconia (YSZ) catalyst and its used in CO and CH4 oxidation. Applied Catalysis A: General, 243, 67–79. https://doi.org/10.1016/S0926-860X(02)00538-0.
Deng, S. C., Zhuang, K., Xu, B. L., Ding, Y. H., Yu, L., & Fan, Y. N. (2016). Promotional effect of iron oxide on the catalytic properties of Fe–MnOx/TiO2 (anatase) catalysts for the SCR reaction at low temperatures. Catalysis Science & Technology, 6, 1772–1778. https://doi.org/10.1039/c5cy01217a.
Duong, L. V., Wood, B. J., & Kloprogge, J. T. (2005). XPS study of basic aluminum sulphate and basic aluminium nitrate. Materials Letters, 59, 1932–1936. https://doi.org/10.1016/j.matlet.2005.02.029.
Fan, J. L., Wei, S. J., Yang, L., Wang, H., Zhong, P., & Zhang, X. (2019). Comparison of the LCOE between coal-fired power plants with CCS and main low-carbon generation technologies: evidence from China. Energy, 176, 143–150. https://doi.org/10.1016/j.energy.2019.04.003.
He, S. L., Li, Q., Zhang, Q., Zhan, Z., & Wang, L. (2019). High efficiency of Ce-modified MnO /TiO -ZrO -based low-temperature selective catalytic reduction denitration catalysts. Journal of Environmental Engineering, 145, 04019087. https://doi.org/10.1061/(ASCE)EE.1943-7870.0001594.
Huang, B. C., Huang, R., Jin, D. J., & Ye, D. Q. (2007). Low temperature SCR of NO with NH3 over carbon nanotubes supported vanadium oxides. Catalysis Today, 126, 279–283. https://doi.org/10.1016/j.cattod.2007.06.002.
Hwang, S., Jo, S. H., Kim, J., Shin, M. C., Chun, H. H., Park, H., & Lee, H. (2016). Catalytic activity of MnOx/TiO2 catalysts synthesized with different manganese precursors for the selective catalytic reduction of nitrogen oxides. Reaction Kinetics, Mechanisms and Catalysis, 117, 583–591. https://doi.org/10.1007/s11144-015-0948-7.
Jin, R. B. (2010). Study on the supported Mn-Ce low-temperature SCR DeNOx catalysts: preparation, reaction mechanism and SO2 tolerance. Dissertation, University of Zhejiang (in Chinese).
Kang, M., Park, E. D., Kim, J. M., & Yie, J. E. (2006). Cu-Mn mixed oxides for low temperature NO reduction with NH3. Catalysis Today, 111, 236–241. https://doi.org/10.1016/j.cattod.2005.10.032.
Kapteijn, F., Langeveld, A. D., Moulijn, J. A., Andreini, A., Vuurman, M. A., Turek, A. M., Jehng, J. M., & Wahs, I. E. (1994). Alumina-supported manganese oxide catalysts: I.Characterization: effect of precursor and loading. Journal of Catalysis, 150, 94–104. https://doi.org/10.1006/jcat.1994.1325.
Kwon, D. W., Park, K. H., & Hong, S. C. (2013). The influence on SCR activity of the atomic structure of V2O5/TiO2 catalysts prepared by a mechanochemical method. Applied Catalysis A: General, 45, 227–235. https://doi.org/10.1016/j.apcata.2012.09.050.
Larrubia, M. A., Ramis, G., & Busea, G. (2000). An FT-IR study of the adsorption of urea and ammonia over V2O5-MoO3-TiO2 SCR catalysts. Applied Catalysis B: Environmental, 27, 145–151. https://doi.org/10.1016/s0926-3373(00)00150-8.
Li, X. S., Yu, D. H., Zhang, W. G., Li, Z. W., Zhang, X. W., & Huang, H. (2013). Effective synthesis of cis-3-hexen-1-yl acetate viatransesterification over KOH/γ-Al2O3: structure and catalytic performance. Applied Catalysis A: General, 455, 1–7. https://doi.org/10.1016/j.apcata.2013.01.015.
Li, L. L., Sun, B., Sun, J. F., Yu, S. H., Ge, C. Y., Tang, C. J., & Dong, L. (2017). Novel MnOx-CeO2 nanosphere catalyst for low-temperature NH3-SCR. Catalysis Communications, 100, 98–102. https://doi.org/10.1016/j.catcom.2017.06.019.
Liu, F. D., & He, H. (2010). Selective catalytic reduction of NO with NH3 over manganese substituted iron titanate catalyst: Reaction mechanism and H2O/SO2 inhibition mechanism study. Catalysis Today, 153, 70–76. https://doi.org/10.1016/j.cattod.2010.02.043.
Liu, F. D., He, H., Ding, Y., & Zhang, C. B. (2009). Effect of manganese substitution on the structure and activity of iron titanate catalyst for the selective catalytic reduction of NO with NH3. Applied Catalysis B: Environmental, 93, 194–204. https://doi.org/10.1016/j.apcatb.2009.09.029.
Lu, Q., Ali, Z., Tang, H., Iqbal, T., Arain, Z., Cui, M. S., Liu, D. J., Li, W. Y., & Yang, Y. P. (2019). Regeneration of commercial SCR catalyst deactivated by arsenic poisoning in coal-fired power plants. Korean Journal of Chemical Engineering, 36, 377–380. https://doi.org/10.1007/s11814-018-0227-9.
Maehida, M., Uto, M., Kurogi, D., & Kijima, T. (2000). MnOx-CeO2 binary oxides for catalytic NOx-sorption at low temperature. Selective reduction of sorbed NOx. Chemistry of Materials, 12, 3158–3164. https://doi.org/10.1021/cm000219c.
Man, Y., Han, Y. L., Hu, Y. S., Yang, S., & Yang, S. Y. (2018). Synthetic natural gas as an alternative to coal for power generation in China: Life cycle analysis of haze pollution, greenhouse gas emission, and resource consumption. Journal of Cleaner Production, 172, 2503–2510. https://doi.org/10.1016/j.jclepro.2017.11.160.
Pena, D. A., Uphade, B. S., Reddy, E. P., & Smiraiotis, P. G. (2004a). Identification of surface species on Titania-supported manganese, chromium, and copper oxide low-temperature SCR catalysts. Journal of Physical Chemistry B, 108, 9927–9936. https://doi.org/10.1021/jp0313122.
Pena, D. A., Uphade, B. S., & Smiraiotis, P. G. (2004b). TiO2-supported metal oxidecatalysts for low-temperature selective catalytic reduction of NO with NH3: I. Evaluation and characterization of first row transition metals. Journal of Catalysis, 221, 421–431. https://doi.org/10.1016/j.jcat.2003.09.003.
Ponce, S., Pena, M. A., & Fierro, J. L. G. (2000). Surface properties and catalytic performance in methane combustion of Sr-substituted lanthanum manganites. Applied Catalysis B: Environmental, 24, 193–205. https://doi.org/10.1016/S0926-3373(99)00111-3.
Qi, G. S., & Yang, R. T. (2003). Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx-CeO2 catalyst. Journal of Catalysis, 217, 434–441. https://doi.org/10.1016/S0021-9517(03)00081-2.
Romeo, M., Bak, K., Fallah, J., Normand, F. L., & Hilaire, L. (1993). XPS study of the reduction of cerium dioxide. Surface and Interface Analysis, 20, 508–512. https://doi.org/10.1002/sia.740200604.
Romero, A., Chacartegui, R., Becerra, J. A., Carvalho, M., & Millar, D. L. (2017). Analysis of the start-up and variable load operation of a combined cycle power plant for off-grid mines. International Journal of Global Warming, 13, 330–335. https://doi.org/10.1504/IJGW.2017.10007769.
Smirniotis, P. G., Sreekanth, P. M., Pena, D. A., & Jenkins, R. G. (2006). Manganese oxide catalysts supported on TiO2, Al2O3, and SiO2: a comparison for low-temperature SCR of NO with NH3. Industrial & Engineering Chemistry Research, 45, 6436–6443. https://doi.org/10.1021/ie060484t.
Sun, P., Guo, R. T., Liu, S. M., Wang, S. X., Pan, W. G., & Li, M. Y. (2017). The enhanced performance of MnO catalyst for NH -SCR reaction by the modification with Eu. Applied Catalysis A-General, 531, 129–138. https://doi.org/10.1016/j.apcata.2016.10.027.
Tang, X. L., Hao, J. M., Yi, H. H., & Li, J. H. (2007). Low-temperature SCR of NO with NH3 over AC/C supported manganese-based monolithic catalysts. Catalysis Today, 126, 406–411. https://doi.org/10.1016/j.cattod.2007.06.013.
Tang, X. F., Li, J. H., Wei, L. S., & Hao, J. M. (2008). MnOx-SnO2 catalysts synthesized by a redox coprecipitation method for selective catalytic reduction of NO by NH3. Chinese Journal of Catalysis, 29, 531–536. https://doi.org/10.1016/S1872-2067(08)60049-2.
Trunschke, A., Hoang, D. L., Radni, J., & Lieske, H. (2000). Influence of lanthana on the nature of surface chromium species in La2O3-modified CrOx/ZrO2 catalysts. Journal of Catalysis, 191, 456–466. https://doi.org/10.1006/jcat.1999.2791.
Wang, H. Q., Cao, S., Fang, Z., Yu, F. X., Liu, Y., Weng, X. L., & Wu, Z. B. (2015). CeO2 doped anatase TiO2 with exposed (001) high energy facets and its performance in selective catalytic reduction of NO by NH3. Applied Surface Science, 330, 245–252. https://doi.org/10.1016/j.apsusc.2014.12.163.
Wang, L. L., Wang, X. Q., Cheng, J. H., Ning, P., & Lin, Y. L. (2018). Coupling catalytic hydrolysis and oxidation on Mn/TiO2-Al2O3 for HCN removal. Applied Surface Science, 439, 213–221. https://doi.org/10.1016/j.apsusc.2018.01.015.
Wei, L., Cui, S. P., Guo, H. X., Ma, X. Y., Wan, Y. Q., & Yu, S. S. (2019). The mechanism of the deactivation of MnOx/TiO2 catalyst for low-temperature SCR of NO. Applied Surface Science, 483, 391–398. https://doi.org/10.1016/j.apsusc.2019.03.280.
Wu, J. C. S., & Cheng, Y. T. (2006). In situ FTIR study of photo catalytic NO reaction on photo catalysts under UV irradiation. Journal of Catalysis, 237, 393–404. https://doi.org/10.1016/j.jcat.2005.11.023.
Wu, Z. B., Jiang, B. Q., Liu, Y., Zhao, W. R., & Guan, B. H. (2007). Experimental study on a low-temperature SCR catalyst based on MnOx/TiO2 prepared by sol-gel method. Journal of Hazardous Materials, 145, 488–494. https://doi.org/10.1016/j.jhazmat.2006.11.045.
Wu, Z. B., Jin, R. B., Liu, Y., & Wang, H. Q. (2008). Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catalysis Communications, 9, 2217–2220. https://doi.org/10.1016/j.catcom.2008.05.001.
Wu, Z. B., Sheng, Z. Y., Liu, Y., Wang, H. Q., & Mo, J. S. (2011). Deactivation mechanism of PtOx/TiO2 photocatalyst towards the oxidation of NO in gas phase. Journal of Hazardous Materials, 185, 1053–1058. https://doi.org/10.1016/j.jhazmat.2010.10.013.
Xie, J. X. (1987). Applications of infrared spectroscopy in organic chemistry and medicinal chemistry. Beijing.
Yan, Z. D., Shi, X. Y., Yu, Y. B., & He, H. (2018). Alkali resistance promotion of Ce-doped vanadium-titanic-based NH3-SCR catalysts. Journal of Environmental Sciences (China), 73, 155–160. https://doi.org/10.1016/j.jes.2018.01.024.
Yu, H. C., Chen, B. Z., Shi, X. C., Sun, X. L., & Li, B. (2008). Investigation of the trivalent-chrome coating on aluminum alloy. Materials Letters, 62, 2828–2831. https://doi.org/10.1016/j.matlet.2008.01.056.
Yun, D., Song, Q., & Yao, Q. (2009). Mechanism and analysis of V2O5-WO3 /TiO2 SCR catalyst deactivation. Coal Conversion, 32, 91–96 (in Chinese).
Zawadzki, J., & Wisniewski, M. (2002). Carbon films as a model material in catalytic NH3/O2 reaction-in situ FTIR study. Fuel Processing Technology, 77, 389–394. https://doi.org/10.1016/S0378-3820(02)00086-3.
Zhang, Y. P., Guo, W. Q., Wang, L. F., Song, M., Yang, L. J., Shen, K., Xu, H. T, & Zhou, C. C. (2015). Characterization and activity of V2O5–CeO2/TiO2–ZrO2 catalysts for NH3-selective catalytic reduction of NOx. Chinese Journal of Catalysis, 36, 1701–1710. https://doi.org/10.1016/S1872-2067(14)60916-0.
Zhang, Q. J., Wu, Y. F., Li, L. L., & Zuo, T. Y. (2018). Sustainable approach for spent V2O5-WO3/TiO2 catalysts management: selective recovery of heavy metal vanadium and production of value-added WO3-TiO2 photocatalysts. ACS Sustainable Chemistry & Engineering, 6, 12502–12506. https://doi.org/10.1021/acssuschemeng.8b03192.
Zhang, W. D., Qi, S. H., Pantaleo, G., & Liotta, L. F. (2019). WO3-V2O5 active oxides for NOx SCR by NH3: preparation methods, catalysts’ composition, and deactivation mechanism-a review. Catalysts, 9, 2–10. https://doi.org/10.3390/catal9060527.
Zhang, D. J., Ma, Z. R., Wang, B. D., Sun, Q., Xu, W. Q., & Zhu, T. (2020). Effects of MOx (M = Mn, Cu, Sb, La) on the V-Mo-Ce/Ti selective catalytic reduction catalysts. Journal of Rare Earths, 38, 157–166. https://doi.org/10.1016/j.jre.2019.02.016.
Zhao, X. T., Mao, L., & Dong, G. J. (2018). Mn-Ce-V-WOx/TiO2 SCR catalysts: catalytic activity, stability and interaction among catalytic oxides. Catalysts, 8, 76–89. https://doi.org/10.3390/catal8020076.
Zhao, S. L., Sun, C., Zhang, Y. Q., Jiao, T. T., Zhang, W. R., Liang, P., & Zhang, H. W. (2019). Determination of mercury occurrence and thermal stability in high ash bituminous coal based on sink-float and sequential chemical extraction method. Fuel, 253, 571–573. https://doi.org/10.1016/j.fuel.2019.05.054.
Funding
This study received financial support from the National Natural Science Foundation of China (51106133, 21237003, 50806041), the Science and Technology Support Program of Jiangsu Province (BE2014682, BY2015061-05), the Yangzhou City Focus on Research and Development Project (YZ2016261, YZ2015043), and the National Spark Program (2015GA690279) and Shanghai Science and Technology Development (15dz1200703, 15110501000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Novelty or Significance
Firstly, the Co-doping could not improve the pore structures of Mn–Ce–Cr series catalysts, but aggravated compaction increasingly on catalyst surfaces.
Secondly, the composite oxide MnxTi1−xO formed on the Mn–Ce–Cr/Al2O3+TiO2 catalyst with higher crystallinity had a better effect on the low-temperature SCR.
Thirdly, the Mn3+ transferred electrons to the Mn4+ due to the oxidation processes of Ce3+ to Ce4+ and Cr3+ to Cr6+, and the Oα/Oβ ratio decreased during SCR process, especially for the Mn–Ce–Cr/Al2O3+TiO2 catalyst, which could promote the low-temperature SCR.
Lastly, the scrapped SCR catalyst from a coal-fired power plant was used as the carrier of the Mn–Ce–Cr catalyst, and its SCR characteristics were found to be much better even at a wider temperature range.
Rights and permissions
About this article
Cite this article
Qi, Y., Shan, X., Wang, M. et al. Study on Low-Temperature SCR Denitration Mechanisms of Manganese-Based Catalysts with Different Carriers. Water Air Soil Pollut 231, 289 (2020). https://doi.org/10.1007/s11270-020-04644-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04644-5