Abstract
Objectives
The dysfunction of mitochondrial respiratory chain induced by cisplatin results in overproduction of reactive oxygen species (ROS) which contributes to kidney injury. The current study aimed to evaluate the effect of a mitochondrial electron transport inhibitors of rotenone (mitochondrial complex I inhibitor) and azoxystrobin (mitochondrial complex III inhibitor), in cisplatin-induced kidney injury.
Methods
In vivo, cisplatin was administered to male C57BL/6J mice by a single intraperitoneal (i.p.) injection (20 mg/kg). Then the mice were treated with or without 200 ppm rotenone in food. Mice were sacrificed after cisplatin administration for 72 h. The serum and the kidney tissues were collected for further analysis. In vitro, mouse proximal tubular cells (mPTCs) were treated with cisplatin (5 µg/mL) and rotenone/azoxystrobin for 24 h. Flow cytometry, Western blotting, and TUNEL staining were used to evaluate the cell injury.
Results
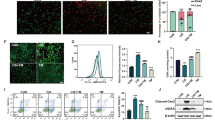
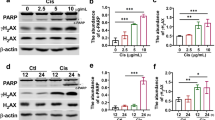
In vivo, rotenone treatment obviously ameliorated cisplatin-induced renal tubular injury evidenced by the improved histology and blocked NGAL upregulation. Meanwhile, cisplatin-induced renal dysfunction shown by the increased levels of serum creatinine (Scr), blood urea nitrogen (BUN), and cystatin C were significantly reduced by rotenone treatment. Moreover, the increments of cleaved caspase-3 and transferase dUTP nick-end labeling (TUNEL)-positive cells were markedly decreased in line with the attenuated mitochondrial dysfunction and oxidative stress after rotenone administration. In vitro, rotenone and azoxystrobin protected against mitochondrial dysfunction, oxidative stress, and renal tubular cell apoptosis induced by cisplatin.
Conclusions
Our results demonstrated that inhibition of mitochondrial activity significantly attenuated cisplatin nephrotoxicity possibly by inhibiting mitochondrial oxidative stress.








Similar content being viewed by others
References
Cohen SM, Lippard SJ (2001) Cisplatin: from DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol 67:93–130
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73(9):994–1007. https://doi.org/10.1038/sj.ki.5002786
Manohar S, Leung N (2018) Cisplatin nephrotoxicity: a review of the literature. J Nephrol 31(1):15–25. https://doi.org/10.1007/s40620-017-0392-z
Peres LA, da Cunha AD Jr (2013) Acute nephrotoxicity of cisplatin: molecular mechanisms. Braz J Nephrol 35(4):332–340. https://doi.org/10.5935/0101-2800.20130052
Che R, Yuan Y, Huang S, Zhang A (2014) Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol Renal Physiol 306(4):F367–F378. https://doi.org/10.1152/ajprenal.00571.2013
Li Y, Ye Z, Lai W, Rao J, Huang W, Zhang X, Yao Z, Lou T (2017) Activation of sirtuin 3 by silybin attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Front Pharmacol 8:178. https://doi.org/10.3389/fphar.2017.00178
Hovater MB, Olteanu D, Welty EA, Schwiebert EM (2008) Purinergic signaling in the lumen of a normal nephron and in remodeled PKD encapsulated cysts. Purinergic Signal 4(2):109–124. https://doi.org/10.1007/s11302-008-9102-6
Dhingra R, Kirshenbaum LA (2014) Regulation of mitochondrial dynamics and cell fate. Circ J 78(4):803–810
Devin A, Rigoulet M (2007) Mechanisms of mitochondrial response to variations in energy demand in eukaryotic cells. Am J Physiol Cell Physiol 292(1):C52–C58. https://doi.org/10.1152/ajpcell.00208.2006
Zsengeller ZK, Ellezian L, Brown D, Horvath B, Mukhopadhyay P, Kalyanaraman B, Parikh SM, Karumanchi SA, Stillman IE, Pacher P (2012) Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J Histochem Cytochem 60(7):521–529. https://doi.org/10.1369/0022155412446227
Tanabe K, Tamura Y, Lanaspa MA, Miyazaki M, Suzuki N, Sato W, Maeshima Y, Schreiner GF, Villarreal FJ, Johnson RJ, Nakagawa T (2012) Epicatechin limits renal injury by mitochondrial protection in cisplatin nephropathy. Am J Physiol Renal Physiol 303(9):F1264–F1274. https://doi.org/10.1152/ajprenal.00227.2012
Bajwa A, Rosin DL, Chroscicki P, Lee S, Dondeti K, Ye H, Kinsey GR, Stevens BK, Jobin K, Kenwood BM, Hoehn KL, Lynch KR, Okusa MD (2015) Sphingosine 1-phosphate receptor-1 enhances mitochondrial function and reduces cisplatin-induced tubule injury. J Am Soc Nephrol 26(4):908–925. https://doi.org/10.1681/ASN.2013121351
Arany I, Safirstein RL (2003) Cisplatin nephrotoxicity. Semin Nephrol 23(5):460–464
Kruidering M, Van de Water B, de Heer E, Mulder GJ, Nagelkerke JF (1997) Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther 280(2):638–649
Chirino YI, Pedraza-Chaverri J (2009) Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol 61(3):223–242. https://doi.org/10.1016/j.etp.2008.09.003
Sun Y, Zhang Y, Zhao D, Ding G, Huang S, Zhang A, Jia Z (2014) Rotenone remarkably attenuates oxidative stress, inflammation, and fibrosis in chronic obstructive uropathy. Mediat Inflamm 2014:670106. https://doi.org/10.1155/2014/670106
Zhang W, Sha Y, Wei K, Wu C, Ding D, Yang Y, Zhu C, Zhang Y, Ding G, Zhang A, Jia Z, Huang S (2018) Rotenone ameliorates chronic renal injury caused by acute ischemia/reperfusion. Oncotarget 9(36):24199–24208. https://doi.org/10.18632/oncotarget.24733
Rajeevan MS, Ranamukhaarachchi DG, Vernon SD, Unger ER (2001) Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods 25(4):443–451. https://doi.org/10.1006/meth.2001.1266
Weidemann A, Bernhardt WM, Klanke B, Daniel C, Buchholz B, Campean V, Amann K, Warnecke C, Wiesener MS, Eckardt KU, Willam C (2008) HIF activation protects from acute kidney injury. J Am Soc Nephrol 19(3):486–494. https://doi.org/10.1681/ASN.2007040419
Yuan Y, Huang S, Wang W, Wang Y, Zhang P, Zhu C, Ding G, Liu B, Yang T, Zhang A (2012) Activation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int 82(7):771–789. https://doi.org/10.1038/ki.2012.188
Oh GS, Kim HJ, Choi JH, Shen A, Choe SK, Karna A, Lee SH, Jo HJ, Yang SH, Kwak TH, Lee CH, Park R, So HS (2014) Pharmacological activation of NQO1 increases NAD(+) levels and attenuates cisplatin-mediated acute kidney injury in mice. Kidney Int 85(3):547–560. https://doi.org/10.1038/ki.2013.330
Oh CJ, Ha CM, Choi YK, Park S, Choe MS, Jeoung NH, Huh YH, Kim HJ, Kweon HS, Lee JM, Lee SJ, Jeon JH, Harris RA, Park KG, Lee IK (2017) Pyruvate dehydrogenase kinase 4 deficiency attenuates cisplatin-induced acute kidney injury. Kidney Int 91(4):880–895. https://doi.org/10.1016/j.kint.2016.10.011
Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV (2011) Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol 22(6):1041–1052. https://doi.org/10.1681/ASN.2010080808
Maimaitiyiming H, Li Y, Cui W, Tong X, Norman H, Qi X, Wang S (2013) Increasing cGMP-dependent protein kinase I activity attenuates cisplatin-induced kidney injury through protection of mitochondria function. Am J Physiol Renal Physiol 305(6):F881–F890. https://doi.org/10.1152/ajprenal.00192.2013
Oh GS, Kim HJ, Shen A, Lee SB, Khadka D, Pandit A, So HS (2014) Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte Blood Press 12(2):55–65. https://doi.org/10.5049/EBP.2014.12.2.55
Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT (2003) Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci 23(34):10756–10764
Alam M, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136(1):317–324
Ichikawa H, Takagi T, Uchiyama K, Higashihara H, Katada K, Isozaki Y, Naito Y, Yoshida N, Yoshikawa T (2004) Rotenone, a mitochondrial electron transport inhibitor, ameliorates ischemia-reperfusion-induced intestinal mucosal damage in rats. Redox Rep 9(6):313–316. https://doi.org/10.1179/135100004225006795
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (Nos. 81670678, 81830020, 81873599, 81530023, 81770690, 81700642, 81670647, and 81570616), the National Key Research and Development Program (No. 2016YFC0906103), and the Natural Science Foundation of Jiangsu Province (No. BK20170150).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest.
Ethical approval
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (IACUC 14030112-2).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Y., Fu, Y., Wang, P. et al. Intervention of mitochondrial activity attenuates cisplatin-induced acute kidney injury. Int Urol Nephrol 51, 1207–1218 (2019). https://doi.org/10.1007/s11255-019-02113-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02113-5