Abstract
Purpose
Conduct a systematic review and meta-analysis of studies to evaluate the association between the use of PDE5I and biochemical recurrence (BCR) after radical prostatectomy (RP).
Methods
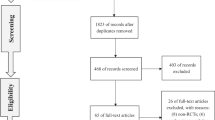
We searched Embase (from 1996 to Feb 2018), PubMed (from 1996 to Feb 2018), and Cochrane library (from 1999 to Feb 2018), then manually searched the reference lists of key retrieved articles. Original studies that reported the risk of postoperative BCR for PDE5I users, as compared with non-PDE5I users, were included. Data including the characteristic of participants, the risk of BCR after RP and key criteria of study quality were collected. The pooled relative risks (RRs) were calculated with random-effects model.
Results
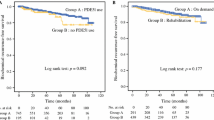
A total of 5 cohort studies and 1 case–control study were conducted for data analysis (a total of 17752 participants). Only 1 cohort study reported adjusted RR greater than 1 (range for all derived RRs, 0.7–1.47). The meta-analysis revealed that the PDE5I users had no higher risk of BCR after RP (RR = 1.04, 95% confidence interval [CI], 0.79–1.36). Sensitivity analysis showed that the remaining pooled RR and 95% CI were not changed significantly by omitting each study. In addition, the 5-year BCR rate had no significant difference between PDE5I users and non-PDE5I users.
Conclusions
Our meta-analysis indicated that PDE5I treatment in men following RP did not increase the risk of BCR. The results preliminarily suggested that the use of PDE5I for erectile dysfunction after RP was oncologically safe. Nevertheless, more large sample cohort studies are needed to validate this conclusion.






Similar content being viewed by others
References
Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, Lin X, Greenfield TK, Litwin MS, Saigal CS, Mahadevan A, Klein E, Kibel A, Pisters LL, Kuban D, Kaplan I, Wood D, Ciezki J, Shah N, Wei JT (2008) Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 358(12):1250–1261. https://doi.org/10.1056/NEJMoa074311
Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, Vardi Y, Wespes E (2010) Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Euro Urol 57(5):804–814. https://doi.org/10.1016/j.eururo.2010.02.020
Michl U, Molfenter F, Graefen M, Tennstedt P, Ahyai S, Beyer B, Budaus L, Haese A, Heinzer H, Oh SJ, Salomon G, Schlomm T, Steuber T, Thederan I, Huland H, Tilki D (2015) Use of phosphodiesterase type 5 inhibitors may adversely impact biochemical recurrence after radical prostatectomy. J Urol 193(2):479–483
Gallina A, Bianchi M, Gandaglia G, Cucchiara V, Suardi N, Montorsi F, Briganti A (2015) A detailed analysis of the association between postoperative phosphodiesterase type 5 inhibitor use and the risk of biochemical recurrence after radical prostatectomy. Euro Urol 68(5):750–753
Loeb S, Folkvaljon Y, Robinson D, Schlomm T, Garmo H, Stattin P (2016) Phosphodiesterase type 5 inhibitor use and disease recurrence after prostate cancer treatment. Euro Urol 70(5):824–828. https://doi.org/10.1016/j.eururo.2015.12.013
Jo JK, Kim K, Lee SE, Lee JK, Byun SS, Hong SK (2016) Phosphodiesterase type 5 inhibitor use following radical prostatectomy is not associated with an increased risk of biochemical recurrence. Ann Surg Oncol 23(5):1760–1767. https://doi.org/10.1245/s10434-015-5059-1
Leapman M, Nguyen H, Cowan J, Porten S, Meng M, Cooperberg M, Carroll P (2016) Phosphodiesterase type 5 inhibitor use is not associated with biochemical recurrence after definitive therapy for prostate cancer. J Urol 1:e1043
Hofer L, Radtke JP, Rapp C, Pahernik S, Teber D, Hohenfellner M, Hadaschik B (2017) Recurrence-free survival after radical prostatectomy and PDE-5 inhibitor intake. Der Urologe Ausg A 56(4):492–496. https://doi.org/10.1007/s00120-016-0267-2
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. Jama 283(15):2008–2012
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2013) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ott Hosp Res Inst. https://doi.org/10.2307/632432
Zhang J, Yu KF (1998) What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama 280(19):1690–1691
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16. https://doi.org/10.1186/1745-6215-8-16
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Leapman MS, Cowan JE, Nguyen HG, Porten SP, Cooperberg MR, Carroll P (2016) Relationship of phosphodiesterase type 5 inhibitor to biochemical recurrence after definitive therapy for prostate cancer. J Clin Oncol Conf. https://doi.org/10.1200/jco.2016.34.2_suppl.119
Poljakovic M, Persson K (2003) Urinary tract infection in iNOS-deficient mice with focus on bacterial sensitivity to nitric oxide. Am J Physiol Ren Physiol 284(1):F22–F31. https://doi.org/10.1152/ajprenal.00101.2002
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867. https://doi.org/10.1038/nature01322
Dennis LK, Lynch CF, Torner JC (2002) Epidemiologic association between prostatitis and prostate cancer. Urology 60(1):78–83
Dennis LK, Dawson DV (2002) Meta-analysis of measures of sexual activity and prostate cancer. Epidemiol (Camb Mass) 13(1):72–79
Nelson WG, De Marzo AM, Isaacs WB (2003) Prostate cancer. N Engl J Med 349(4):366–381. https://doi.org/10.1056/NEJMra021562
Bian K, Ghassemi F, Sotolongo A, Siu A, Shauger L, Kots A, Murad F (2012) NOS-2 signaling and cancer therapy. IUBMB Life 64(8):676–683. https://doi.org/10.1002/iub.1057
Qian CN, Takahashi M, Kahnoski R, Teh BT (2003) Effect of sildenafil citrate on an orthotopic prostate cancer growth and metastasis model. J Urol 170(3):994–997. https://doi.org/10.1097/01.ju.0000080321.99119.df
Zenzmaier C, Sampson N, Pernkopf D, Plas E, Untergasser G, Berger P (2010) Attenuated proliferation and trans-differentiation of prostatic stromal cells indicate suitability of phosphodiesterase type 5 inhibitors for prevention and treatment of benign prostatic hyperplasia. Endocrinology 151(8):3975–3984. https://doi.org/10.1210/en.2009-1411
Chavez AH, Coffield KS, Rajab MH, Jo C (2013) Incidence rate of prostate cancer in men treated for erectile dysfunction with phosphodiesterase type 5 inhibitors: retrospective analysis. Asian J Androl 15(2):246–248. https://doi.org/10.1038/aja.2012.162
Giles GG, Severi G, English DR, McCredie MR, Borland R, Boyle P, Hopper JL (2003) Sexual factors and prostate cancer. BJU Int 92(3):211–216
Leitzmann MF, Platz EA, Stampfer MJ, Willett WC, Giovannucci E (2004) Ejaculation frequency and subsequent risk of prostate cancer. Jama 291(13):1578–1586. https://doi.org/10.1001/jama.291.13.1578
Fokas E, McKenna WG, Muschel RJ (2012) The impact of tumor microenvironment on cancer treatment and its modulation by direct and indirect antivascular strategies. Cancer Metastasis Rev 31(3–4):823–842. https://doi.org/10.1007/s10555-012-9394-4
Jerzak M, Kniotek M, Mrozek J, Gorski A, Baranowski W (2008) Sildenafil citrate decreased natural killer cell activity and enhanced chance of successful pregnancy in women with a history of recurrent miscarriage. Fertil Steril 90(5):1848–1853. https://doi.org/10.1016/j.fertnstert.2007.08.043
Hall SA, Link CL, Hu JC, Eggers PW, McKinlay JB (2009) Drug treatment of urological symptoms: estimating the magnitude of unmet need in a community-based sample. BJU Int 104(11):1680–1688. https://doi.org/10.1111/j.1464-410X.2009.08686.x
Li J, Shi Q, Pu C, Tang Y, Bai Y, Yuan H, Li X, Dong Q, Wei Q, Yuan J, Han P (2014) Phosphodiesterase type 5 inhibitors for the treatment of post-nerve sparing radical prostatectomy erectile dysfunction in men. Sci Rep 4:5801. https://doi.org/10.1038/srep05801
Acknowledgements
This study was supported by Grant Nos. 81770703 and 81470927 from the National Natural Science Foundation of China and Grant No. 16PI294 from Project of the Health and Family Planning Committee of Sichuan Province.
Author information
Authors and Affiliations
Contributions
Q He: Project development, Data analysis, Quality assessment, Manuscript writing. BH Liao: Data analysis, Quality assessment, Manuscript editing. KW Xiao: Data collection, Chart production. L Zhou: Protocol development. SJ Feng: Data collection. H Li: Manuscript editing. KJ Wang: Project development, Data management, Manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists.
Ethical approval
This meta-analysis was based on published studies. Therefore, ethical approval and informed consent are not required for this type of study.
Rights and permissions
About this article
Cite this article
He, Q., Liao, BH., Xiao, KW. et al. Is there a relationship between phosphodiesterase type 5 inhibitors and biochemical recurrence after radical prostatectomy: a systematic review and meta-analysis. Int Urol Nephrol 50, 2113–2121 (2018). https://doi.org/10.1007/s11255-018-1982-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-1982-y