Abstract
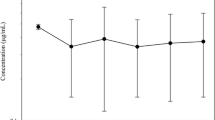
This study investigated the pharmacokinetics of pentoxifylline (PTX) and its 5-hydroxyhexyl metabolite (M-I) after single-dose intravenous (IV) administration (10 mg/kg) of PTX in six healthy cattle. The safety of PTX was evaluated by clinical observation and biochemical analysis. Plasma concentrations of PTX and M-I were simultaneously determined by reverse-phase high performance liquid chromatography. Pharmacokinetic parameters were calculated using non-compartmental methods. Salivation and discomfort were observed for 2 h following the drug administration. Serum direct bilirubin, total bilirubin, and phosphorus levels at 24 h following the drug administration were significantly different from the control values (0 h) (P < 0.05). Pharmacokinetic variables of PTX were characterized by a short terminal elimination half-life (1.05 ± 0.19 h), a large volume of distribution (6.30 ± 1.76 L/kg), and high total body clearance (5.31 ± 1.27 L/h/kg). The mean ratio between the area under the concentration-time curves of M-I and PTX was 1.34. These results indicate that single-dose administration of PTX at 10 mg/kg IV in cattle resulted in therapeutic concentrations similar to those observed in humans and horse. However, further studies are necessary to determine the safety and pharmacokinetics following repeated administrations of PTX.

Similar content being viewed by others
References
Adcock, K.G., Kyle, P.B., Deaton, J.S., Olivier, J.H., and Hogan, S.M., 2007. Pharmacokinetics of intranasal and intratracheal pentoxifylline in rabbits, Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 27, 200–206.
Ambrus, J.L., Stadler, S., and Kulaylat, M., 1995. Hemorrheologic effects of metabolites of pentoxifylline (Trental), Journal of Medicine, 26, 65–75.
Aviado, D.M., and Porter, J.M., 1984. Pentoxifylline: a new drug for the treatment of intermittent claudication; mechanism of action, pharmacokinetics, clinical efficacy and adverse effects, Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 4, 297–306.
Barton, M.H., Ferguson, D., Davis, P.J., and Moore, J.N., 1997. The effects of pentoxifylline infusion on plasma 6-keto-prostaglandin F1α and ex vivo endotoxin-induced tumour necrosis factor activity in horses, Journal of Veterinary Pharmacology and Therapeutics, 20, 487–492.
Barton, M.H., and Moore, J.N., 1994. Pentoxifylline inhibits mediator synthesis in an equine in vitro whole blood model of endotoxemia, Circulatory Shock, 44, 216–220.
Beermann, B., Ings, R., Månsby, J., Chamberlain, J., and McDonald, A., 1985. Kinetics of intravenous and oral pentoxifylline in healthy subjects, Clinical Pharmacology & Therapeutics, 37, 25–28.
Bessler, H., Gilgal, R., Djaldetti, M., and Zahavi, I., 1986. Effect of pentoxifylline on the phagocytic activity, cAMP levels, and superoxide anion production by monocytes and polymorphonuclear cells, Journal of Leukocyte Biology, 40, 747–754.
Cakmak, K.S., Cakmak, A., Gonul, M., Kilic, A., and Gul, U., 2012. Pentoxifylline use in dermatology, Inflammation & Allergy-Drug Targets, 11, 422–432.
Crisman, M.V., Wilcke, J.R., Correll, L.S., and Irby, M.H., 1993. Pharmacokinetic disposition of intravenous and oral pentoxifylline in horses, Journal of Veterinary Pharmacology and Therapeutics, 16, 23–31.
De Boever, S., Baert, K., De Backer, P., and Croubels, S., 2005. Pharmacokinetics and oral bioavailability of pentoxyfylline in broiler chickens, Journal of Veterinary Pharmacology and Therapeutics, 28, 575–580.
Harris, E., Schulzke, S.M., and Patole, S.K., 2010. Pentoxifylline in preterm neonates, Pediatric Drugs, 12, 301–311.
Hinze, H.J., 1972. Pharmacokinetics of 3, 7-dimethyl-1-(5-oxo-hexyl)-xanthine (BL 191) in man, Arzneimittel-Forschung, 22, 1492.
Honess, D.J., Dennis, I.F., and Bleehen, N.M., 1993. Pentoxifylline: its pharmacokinetics and ability to improve tumour perfusion and radiosensitivity in mice, Radiotherapy and Oncology, 28, 208–218.
Italiya, K.S., Sharma, S., Kothari, I., Chitkara, D., and Mittal, A., 2017. Simultaneous estimation of lisofylline and pentoxifylline in rat plasma by high performance liquid chromatography-photodiode array detector and its application to pharmacokinetics in rat, Journal of Chromatography B, 1061, 49–56.
Jerzsele, A., 2012. Comparative Veterinary Pharmacokinetics, In: Noreddin A (ed), Readings in Advanced Pharmacokinetics—Theory, Methods and Applications, IntechOpen. https://doi.org/10.5772/1982.
Kwong, E.C., Chen, F.C., and Young, L.M., 1989. Urinary excretion of pentoxifylline and its metabolites by standardbred mares, Canadian Journal of Veterinary Research, 53, 147–153.
Landoni, M.F., Cunningham, F.M., and Lees, P., 1996. Pharmacokinetics and pharmacodynamics of tolfenamic acid in calves, Research in Veterinary Science, 61, 26–32.
Liska, D.A., Akucewich, L.H., Marsella, R., Maxwell, L.K., Barbara, J.E., and Cole, C.A., 2006. Pharmacokinetics of pentoxifylline and its 5-hydroxyhexyl metabolite after oral and intravenous administration of pentoxifylline to healthy adult horses, American Journal of Veterinary Research, 67, 1621–1627.
Luke, D.R., Rocci, M.L., and Hoholick, C., 1986. Inhibition of pentoxifylline clearance by cimetidine, Journal of Pharmaceutical Sciences, 75, 155–157.
Malátková, P., and Wsól, V., 2014. Carbonyl reduction pathways in drug metabolism, Drug Metabolism Reviews, 46, 96–123.
Marsella, R., Nicklin, C.F., Munson, J.W., and Roberts, S.M., 2000. Pharmacokinetics of pentoxifylline in dogs after oral and intravenous administration, American Journal of Veterinary Research, 61, 631–637.
Nicklasson, M., Björkman, S., Roth, B., Jönsson, M., and Höglund, P., 2002. Stereoselective metabolism of pentoxifylline in vitro and in vivo in humans, Chirality, 14, 643–652.
Paap, C.M., Simpson, K.S., Horton, M.W., Schaefer, K.L., Lassman, H.B., and Sack, M.R., 1996. Multiple-dose pharmacokinetics of pentoxifylline and its metabolites during renal insufficiency, Annals of Pharmacotherapy, 30, 724–729.
Peterson, T.C., Peterson, M.R., Wornell, P.A., Blanchard, M.G., and Gonzalez, F.J., 2004. Role of CYP1A2 and CYP2E1 in the pentoxifylline ciprofloxacin drug interaction, Biochemical Pharmacology, 68, 395–402.
Radostitis, O.M., Gay, C.C., Blood, D.C., and Hinchcliff, K.W., 2007. Veterinary Medicine: A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. 10th ed, (W. B. Saunders, London), 2048–2050.
Rames, A., Poirier, J.M., LeCoz, F., Midavaine, M., Lecocq, B., Grange, J.D., Poupon, R., Cheymol, G., and Jaillon, P., 1990. Pharmacokinetics of intravenous and oral pentoxifylline in healthy volunteers and in cirrhotic patients, Clinical Pharmacology & Therapeutics, 47, 354–359.
Raoul, J.M., Peterson, M.R., and Peterson, T.C., 2007. A novel drug interaction between the quinolone antibiotic ciprofloxacin and a chiral metabolite of pentoxifylline, Biochemical Pharmacology, 74, 639–646.
Rees, C., Boothe, D.M., Boeckh, A., Wilkie, S., Esparza, T., and Green, R., 2003. Dosing regimen and hematologic effects of pentoxifylline and its active metabolites in normal dogs, Veterinary Therapeutics: Research in Applied Veterinary Medicine, 4, 188–196.
Regenthal, R., Krueger, M., Koeppel, C., and Preiss, R., 1999. Drug levels: therapeutic and toxic serum/plasma concentrations of common drugs, Journal of Clinical Monitoring and Computing, 15, 529–544.
Samlaska, C.P., and Winfield, E.A., 1994. Pentoxifylline, Journal of the American Academy of Dermatology, 30, 603–621.
Sivapathasundaram, S., Magnisali, P., Coldham, N.G., Howells, L.C., Sauer, M.J., and Ioannides, C., 2001. A study of the expression of the xenobiotic-metabolising cytochrome P450 proteins and of testosterone metabolism in bovine liver, Biochemical Pharmacology, 62, 635–645.
Smith, R.V., Waller, E.S., Doluisio, J.T., Bauza, M.T., Puri, S.K., Ho, I., and Lassman, H.B., 1986. Pharmacokinetics of orally administered pentoxifylline in humans, Journal of Pharmaceutical Sciences, 75, 47–52.
Sykes, J.E., and Papich, M.P., 2014. Antiviral and Immunomodulatory Drugs, In: Sykes, J.E. (ed), Canine and Feline Infectious Diseases (Elsevier Saunders, St. Louis, Missouri 63043), 54–65.
Szotáková, B., Baliharová, V., Lamka, J., Nožinová, E., Wsól, V., Velık, J., Machala, M., Neča, J., Souček, P., Šusová, S., and Skálová, L., 2004. Comparison of in vitro activities of biotransformation enzymes in pig, cattle, goat and sheep, Research in Veterinary Science, 76, 43–51.
Toutain, P.L., Ferran, A., and Bousquet-Mélou, A., 2010. Species differences in pharmacokinetics and pharmacodynamics, Handbook of Experimental Pharmacology, 199, 19–48.
Wyska, E., 2010. Pharmacokinetic-pharmacodynamic modeling of methylxanthine derivatives in mice challenged with high-dose lipopolysaccharide, Pharmacology, 85, 264–271.
Yang, Z., Chen, M., and Nadler, J.L., 2005. Lisofylline: a potential lead for the treatment of diabetes, Biochemical Pharmacology, 69, 1–5.
Acknowledgements
This study was presented in abstract form as a poster presentation in the 3rd International Convention of Pharmaceuticals and Pharmacies, Istanbul, Turkey, 26–29 April 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal experiment was conducted in accordance with the procedure of Committee on Animal Use Ethics (Approval No. 2015/96).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Uney, K., Tras, B., Corum, O. et al. Pharmacokinetics of pentoxifylline and its 5-hydroxyhexyl metabolite following intravenous administration in cattle. Trop Anim Health Prod 51, 435–441 (2019). https://doi.org/10.1007/s11250-018-1710-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-018-1710-8