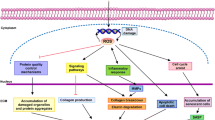
Abstract
Photosensitization is severe dermatitis or oxidative/chemical changes in the epidermal tissues activated by the light-induced excitation of molecules within the tissue. It is a series of reactions mediated through light receptors and is more common when the plant-produced metabolites are heterocyclic/polyphenols in nature. The areas affected are exposed body parts and mostly non-pigmented areas with least ultraviolet protection. Similarly, cellular alteration also occurs in the affected animal’s dermal tissues and body parts and grazing animals by the accumulation and activation of photodynamic molecules. Photo-oxidation can also occur within the plant due to the generation of reactive oxygen species causing damage and degradation in the form of free radicals and DNA. During the last few decades, many new tropical grass species have been introduced in the grazing lands which are genetically modified, and the animals grazing on them are facing various forms of toxicity including photosensitization. The plant’s secondary metabolites/drugs may cause toxicity when bacteria, viral agents, fungi (Pithomyces chartarum), or neoplasia injures the liver and prevents the phylloerythrin excretion. All these may disturb the liver enzymes and blood profile causing a decrease in weight and production (wool and milk etc.) with severe dermal, digestive, and nervous problems. Recent advancements in OMICS (cellomics, ethomics, metabolomics, metabonomics, and glycomics) have enabled us to detect and identify the plants’ secondary metabolites and changes in the animal’s physiology and histopathology as a causative of photosensitivity. The review focuses on types of photosensitization, reasons, secondary metabolic compounds, chemistry, and environmental effect on plants.











Similar content being viewed by others
References
Alexandru B.; Susanne K. S.; Lavanya M.; Tatjana I. K.; Cristian Ne.; Barbara N.; Milos F.; Peter M.; Peter W. R. and Michael J. M. F. Photosensitization in porphyrias and photodynamic therapy involves trpa1 and trpv1. Journal of neuroscience. 2016, 36 (19) 5264–5278.
Atta ur Rehman. Studies in natural products chemistry. Vol. 23. Bioactive natural products (Part D). Elsevier. 2000. Amsterdam, Netherland.
Blood, D.C.; Radostits, O.M. Diseases caused by protozoa. In: __. Veterinary clinic. 7. ed. Rio de Janeiro: Guanabara, 1991, Ch. 25, p. 823–850.
Bourke C.A. The effect of shade, shearing and wool type in the protection of Merino sheep from Hypericum perforatum (St. John’s wort) poisoning. Aust. Vet. J. 2003, 81; 494–498.
Bourke C.A., White J.G. Reassessment of the toxicity of Hypericum perforatum (St. John’s wort) for cattle. Aust. Vet. J. 2004; 82:707–710.
Campbell, W.M.; Dombroski, G.S.; Sharma, I.; Partridge, A.C.; Collett, M.G. Photodynamic chlorophyll metabolites, including phytoporphyrin (phylloerythrin), in the blood of photosensitive livestock: Overview and measurement. N. Z. Vet. J. 2010, 58 (3), 146–154.
Cao Y.; S.M. Colegate; J.A. Edgar. Persistence of echimidine, a hepatotoxic pyrrolizidine alkaloid, from honey into mead. Short Communication. Journal of Food Composition and Analysis. 2013, Vol. 29, P: 106–109.
Chenyu Wu, Sivaprakash S. Jiangtao X., Jian Z. and Cyrille B. Chlorophyll a crude extract: efficient photo-degradable photocatalyst for PET-RAFT polymerization. Chemical Communications. 2017, 53, 12560–12563.
C.H.S. De Oliveira, J.D. Barbosa, C.M.C. Oliveira, E. Bastianetto, M.M. Melo, M. Haraguchi, L.G.L. Freitas, M.X. Silva, R.C. Leite. Hepatic photosensitization in buffaloes intoxicated by Brachiaria decumbens in Minas Gerais state, Brazil, Toxicon. 2013, 73:121-129.
Croteau, R.; Kutchman, T.M.; Lewis, N.G. Natural products (secondary metabolites). In Biochemistry and Molecular Biology of Plants; American Society of Plant Biologists: Rockville, 2000.
Harborne, J. B. Introduction to Ecological Biochemistry, 4th ed. Academic Press, London, pp. 384. 1993.
Field, B.; Jordan, F.; Osbourn, A. First encounters—Deployment of defence-related natural products by plants. N. Phytol. 2006, 172, 193–207.
Franco D, Lin T, Leder J. Bovine congenital erythropoietic porphyria. Comp Cont Educ Pract 1992, 14:822–5.
Gracindo, C. V.; Louvandini, H.; Riet-Correa, F.; Barbosa-Ferreira, M.; de Castro, M. B. Performance of sheep grazing in pastures of Brachiaria decumbens, Brachiaria brizantha, Panicum maximum, and Andropogon gayanus with different protodioscin concentrations. Trop. Anim. Health Prod. 2014, 1–5.
Gupta, R.C. Veterinary Toxicology—Basic and Clinical Principles, 2nd ed.; Elsevier: London, 2012.
Gupta, V.; Su, Y.S.; Wang, W.; Kardosh, A.; Liebes, L.F.; Hofman, F.M.; Schontha, A.H.; Chen, T.C. Enhancement of glioblastoma cell killing by combination treatment with temozolomide and tamoxifen or hypericin. Neurosurg. Focus 2006, 20, E20.
Hargis, A. M. The integumentary system. In: Carlton, W.W.; Mcgavin, M.D. Pathology Veterinary surgeon. Porto Alegre: Artmed, 1998, Ch. 11, p. 486–539.
Huxley J. N.; Lloyd E. L.; Parker C. S.; Woolf J. R. and Strugnell B. W. Congenital erythropoietic porphyria in a longhorn calf. Vet Rec. 2009, 165 (23): 694–5.
Kessel, D. and Smith, K. Photosensitization with derivatives of Chrolophyll. Photochemistry and Photobiology. 1989, 49: 157–160.
Knight, A.P.; Walter R. G. A Guide to Plant Poisoning of Animals in North America; Teton New Media: Jackson, 2001.
Launchbaugh, K.L. Biochemical Aspects of Grazing Behavior; CAB International: Wallingford, Oxfordshire, 1996, pp. 159–184.
Lecha M.; Puy H. and Deybach J. C. Erythropoietic protoporphyria. Orphanet J Rare Dis. 2009, 4:19.
McAloon Conor G., Michael L. Doherty, Henry O’Neill, Michael Badminton and Eoin G. Ryan. Bovine congenital erythropoietic protoporphyria in a crossbred limousin heifer in Ireland. 2015, 68:15.
Montiero-Riveiere, N. The Integument. Dellman’s Textbook of Veterinary Histology, 6th ed.; Blackwell Press: Ames, 2006.
Pereira, F. E. L. General etiopathogenesis of lesions. In: FILHO, G. B. Bogliolo pathology. 6. ed. Rio de Janeiro: Guanabara, 2000, Ch. 3, p. 19–37.
Perez A. J., Syeda M. Hussain, Łukasz Pecio, Mariusz Kowalczyk, Valdo R. Herling, and Anna Stochmal. Ultrahigh-performance liquid chromatography −high-resolution quadrupole time of flight mass spectrometry-based metabolomics reveals key differences between Brachiaria decumbens and B. brizantha, two similar pastures with different toxicities. J. Agric. Food Chem. 2016, Vol. 64, P: 4686−4694.
Peter P. Fu, Qingsu X.; Ge L. and Ming W. C. Pyrrolizidine Alkaloids—Genotoxicity, Metabolism Enzymes, Metabolic Activation and Mechanisms. Drug Metabolism Reviews. 2004, Vol. 36 (1), pp. 1–55.
Pickett, J.A. New synthesis: Chemical ecology and sustainable food production. J. Chem. Ecol. 2012, 38, 1071–1071.
Provenza, F.D.; Pfister, J.A.; Cheney, C.D. Mechanisms of learning in diet selection with reference to phyto toxicosis in herbivores. J. Range Manag. 1992, 45, 36–45.
Quinn, J. C.; Kessell, A.; Weston, L. A. Secondary plant products causing photo-sensitization in grazing herbivores: Their structure, activity and regulation. Int. J. Mol. Sci. 2014, 15, 1441−1465.
Radostits O.M., Gay C., Hinch C. K.W., Constable P. D. Veterinary Medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats. 10th ed. London: Vet Med; 2007, P. 1548–51.
Sanderson B.J.S. and A.M. Clark. Micronuclei in adult and foetal mice exposed in vivo to heliotrine, urethane, monocrotaline and benzidine. Mutation Research, 1993, 285, 27–33. Elsevier Science Publishers, Bedford Park SA 5042, Australia.
Scheie, E.; Flaoyen, A.; Moan, J.; Berg, K. Phylloerythrin: Mechanisms for cellular uptake and location, photo-sensitization and spectroscopic evaluation. N. Z. Vet. J. 2002, 50, 104–110.
Schild, A.L.; A.C. Motta; F. Riet-Correa; F.C. Karam and F.B. Greeco. Ch. 26, Photosensitization in Cattle in Southern. Brazil. In F. Riet-Correa, J. Pfister, A.L. Schild, and T. Wierenga, eds., Poisoning by Plants, Mycotoxins, and Related Toxins, pp. 68–72. CAB International, Wallingford 2011.
Schoental R. Pancreatic Islet-Cell and Other Tumors in Rats Given Heliotrine, a Monoester Pyrrolizidine Alkaloid, and Nicotinamide. American association for cancer research. August 1975. 35 (8), p: 2020–2024.
Smith, E.; Kiss, F.; Porter, R.M.; Anstey, A.V. A review of UVA-mediated photosensitivity disorders. Photochem. Photobiol. Sci. 2012, 11, 199–206.
Stannard, A. A. Skin diseases - dermatopathies. In: Smith, B. P. Internal medicine of large animals. São Paulo: Manole, 1994, (2) Ch. 35, p. 1061–1117.
Stegelmeier B.L. Equine photosensitization. Clin. Techn. Equine Pract. 2002; 1:81–88.
Stegelmeier, B.L.; Colegate, S.M.; Brown, A.W. Review on Dehydropyrrolizidine Alkaloid Toxicity, Cytotoxicity, and Carcinogenicity. Toxins 2016, 8:356.
Step DL, Smith RA. Review Non-respiratory diseases of stocker cattle. Vet Clin North Am Food Anim Pract. 2006, 22 (2): 413–34.
Tao Y.; Lan, L. F.; Hong, L. C.; Guo, Y. Z.; Hau, M. S.; Yi,-N. Tang.; Lin Zhu.; Chu Chu.; Zhong-Z. Z. and Hu-B. Chen. Comparative analysis of diosgenin in Dioscorea species and related medicinal plants by UPLC-DAD-MS. BMC Biochemistry 2014, 15:19.
Takeda, Yuiko & Konuma, Hirotaka & Uchiyama, Sadao & Saito, Yukio. Microbial Formation of Pheophorbide a and Pyropheophorbide a in “Takana” during Brining and Fermentation. Food hygiene and Safety Science (Shokuhin Eiseigaku Zasshi). 1989, 30 (3); 228–232.
Tapper, B. A.; Lohrey E.; Hove E. L.; Allison, R. M. Photosensitivity from chlorophyll derived pigments. Journal of the Science of Food and Agriculture 1975, (26) 277–84,
The New York Botanical Garden. January, 2014. http://sweetgum.nybg.org/science/vh/specimen_details.php?irn=2952420
Truyers I, Ellis K. Case report: bovine congenital erythropoietic protoporphyria in a pedigree Limousin herd. Livestock. 2013, 18:30–3.
Wallaart, T.E.; Pras, N.; Quax, W.J. Isolation and identification of di-hydro artemisinic acid hydroperoxide from Artemisia annual: A novel biosynthetic precursor of artemisinin. J. Nat. Prod. 1999a, 62, 1160–1162.
Wallaart, T.E.; Pras, N.; Quax, W.J. Seasonal variations of artemisinin and its biosynthetic precursors in tetraploid Artemisia annual plants compared with the diploid wild-type. Planta Med. 1999b, 65, 723–728.
Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 6 (39), 283–297.
Wink, M. Biochemistry of Plant Secondary Metabolism. Annual Plant Reviews Vol. 2. 1999. Sheffield Academic Press, Sheffield.
Wink, M. and Hartmann, Localisation of the enzymes of quinolizidine alkaloid biosynthesis in leaf chloroplast of Lzcpinus polyphyllous. Plant Physiology, 1982, 70, 74–77.
Wink, M. Introduction: Biochemistry, role and biotechnology of secondary metabolites. In: Wink, M. (ed.) Annual Plant Reviews, Vol. 40. Biochemistry of Plant Secondary Metabolism, 2nd edition. Blackwell Publishing, UK. 2010.
Wink, M. Mode of action of alkaloids. In: Roberts, M.F. and Wink, M. (eds) Alkaloids: Biochemistry, Ecology, and Medicinal Applications. Plenum Press, New York, pp. 265–298. 1998.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hussain, S.M., Herling, V.R., Rodrigues, P.H.M. et al. Mini review on photosensitization by plants in grazing herbivores. Trop Anim Health Prod 50, 925–935 (2018). https://doi.org/10.1007/s11250-018-1583-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-018-1583-x