Abstract
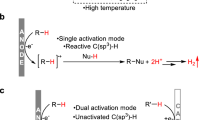
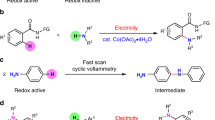
Joined electrolysis of arenes (benzene or coumarin derivatives) and diethyl-H-phosphonate (EtO)2P(O)H in the presence of [CoCl2(bpy)] catalyst (5%) in an ethanol-aqueous solution in reductive conditions allows obtaining the desired products in a single step by aromatic C–H bonds phosphonation with yields up to 70%. The only by-product is hydrogen; the reaction proceeds at room temperature and does not require specially added reducing agents and oxidants or other initiators. Radical mechanism has been confirmed for the catalytic reaction proceeding via bicobalt phosphonates with Co–P bond, the structure of which also has been identified.







Similar content being viewed by others
References
Constable DJC, Dunn PJ, Hayler JD, Humphrey GR, Leazer Jr JL, Linderman LK, Manley J, Pearlman BA, Wells A, Zaks A, Zhang TY (2007) Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem 9:411–420
Fabry DC, Rueping M (2016) Merging visible light photoredox catalysis with metal catalyzed C–H activations: on the role of oxygen and superoxide ions as oxidants. Acc Chem Res 49:1969–1979
Chupakhin ON, Charushin VN (2016) Recent advances in the field of nucleophilic aromatic substitution of hydrogen. Tetrahedron Lett 57:2665–2672
Rodesly F, Oble J, Poli G (2017) Metal-catalyzed CH activation/functionalization: the fundamentals. J Mol Catal A 426:275–296
Budnikova YH, Sinyashin OG (2015) Phosphorylation of C–H bonds of aromatic compounds using metals and metal complexes. Russ Chem Rev 84:917–951
Mikhaylov DY, Budnikova YH (2013) Fluoroalkylation of organic compounds. Russ Chem Rev 82:835–864
Odinets IL, Vinogradova NM, Lyssenko KA, Petrovskii PV, Mastryukova TA, Röschenthaler GV (2006) Diastereoselective cycloalkylation of phosphoryl and thiophosphoryl acetonitriles by α, ψ-dihalogenalkanes under phase transfer catalysis conditions. Heteroat Chem 17:13–21
Trofimov BA, Arbuzova SN, Gusarova NK (1999) Phosphine in the synthesis of organophosphorus compounds. Russ Chem Rev 68:215–228
Lebel H, Morin S, Paquet V (2003) Alkylation of phosphine boranes by phase-transfer catalysis. Org Lett 5:2347–2349
Odinets IL, Matveeva EV (2012) The application of green chemistry methods in organophosphorus synthesis. Russ Chem Rev 81:221–238
Budnikova YH, Gryaznova TV, Grinenko VV, Dudkina YB, Khrizanforov MN (2017) Eco-efficient electrocatalytic C–P bond formation. Pure Appl Chem 89:311–330
Budnikova YH, Krasnov SA, Gryaznova TV, Tomilov AP, Turigin VV, Magdeev IM, Sinyashin OG (2008) “Green” ways of phosphorus compounds preparation. Phosphorus Sulfur Silicon Relat Elem 183:513–518
Budnikova YH, Yakhvarov DG, Sinyashin OG (2005) Electrocatalytic eco-efficient functionalization of white phosphorus. J Organomet Chem 690:2416–2425
Li C-J (2016) Exploration of new chemical reactivities for sustainable molecular transformations. Chem 1(3):423–437
Feng C-G, Ye M, Xiao K-J, Li S, Yu J-Q (2013) Pd(II)-catalyzed phosphorylation of aryl C–H bonds. J Am Chem Soc 135(25):9322–9325
Chen P, Sun Y, Wu Y, Liu L, Zhu J, Zhao Y (2017) A theoretical study on the mechanism of ruthenium(II)-catalyzed phosphoryl-directed ortho-selective C–H bond activations: the phosphoryl hydroxy group triggered Ru(II)/Ru(0) catalytic cycle. Org Chem Front 4:1482–1492
Liu L, Yuan H, Fu T, Wang T, Gao X, Zeng Z, Zhu J, Zhao Y (2014) Double role of the hydroxy group of phosphoryl in palladium(II)-catalyzed ortho-olefination: a combined experimental and theoretical investigation. J Org Chem 79(1):80–87
Kosolapoff GM, Maier L (1972) Organic phosphorus compounds. Wiley, New York
Corbridge DEC (2013) Phosphorus: chemistry, biochemistry and technology. CRC Press, London
Swaminathan S, Narayanan KV (1971) Rupe and meyer-schuster rearrangements. Chem Rev 71:429–438
Bhattacharya AK, Thyagarajan G (1981) Michaelis-arbuzov rearrangement. Chem Rev 81:415–430
Kostova I (2005) Synthetic and natural coumarins as cytotoxic agents. Curr Med Chem 5:29–46
Venugopala KN, Rashmi V, Odhav B (2013) Review on natural coumarin lead compounds for their pharmacological activity. Biomed Res Int 2013:1–14
Budzisz E, Brzezinska E, Krajewska U, Rozalski M (2003) Cytotoxic effects, alkylating properties and molecular modelling of coumarin derivatives and their phosphonic analogues. Eur J Med Chem 38:597–603
Engel R, Cohen JI (2003) Synthesis of carbon–phosphorus bonds. CRC Press, Boca Raton
Tavs P (1970) Reaktion von Arylhalogeniden mit Trialkylphosphiten und Benzolphosphonigsäure-dialkylestern zu aromatischen Phosphonsäureestern und Phosphinsäureestern unter Nickelsalzkatalyse. Eur J Inorg Chem 103:2428–2436
Connor JA, Jones AC, Price R (1980) Copper (II) ethanoate-assisted phosphonation of aryl halides. J Chem Soc Chem Commun 4:137–138
Hall N, Price R (1979) The copper-promoted reaction of o-halogenodiarylazo-compounds with nucleophiles. Part 1. The copper-promoted reaction of o-bromodiarylazo-compounds with trialkyl phosphites. A novel method for the preparation of dialkyl arylphosphonates. J Chem Soc Perkin Trans 1:2634–2641
Hirao T, Masunaga T, Oshiro Y, Agawa T (1981) A novel synthesis of dialkyl arenephosphonates. Synthesis 1981:56–57
Hirao T, Masunaga T, Yamada N, Oshiro Y, Agawa T (1982) Palladium-catalyzed new carbon-phosphorus bond formation. Bull Chem Soc Jpn 55:909–913
Battagia S, Vyle S (2003) Novel methodology for the preparation and purification of oligonucleotides incorporating phosphorothiolate termini. Tetrahedron Lett 44:861–863
Obrycki R, Griffin CE (1968) Phosphonic acids and esters. XIX. Synthesis of substituted phenyl-and arylphosphonates by the photoinitiated arylation of trialkyl phosphites. J Org Chem 33:632–636
Bunnett JF, Creary X (1974) Photostimulated condensation of aryl iodides with potassium dialkyl phosphites to form dialkyl arylphosphonates. J Org Chem 39:3612–3614
Jason EF, Fields EK (1962) Free-radical phosphonation of aromatic compounds. J Org Chem 27:1402–1405
Kottman H, Skarzewski J, Effenberger F (1987) Oxidative phosphonylierung von aromaten mit cerammoniumnitrat. Synthesis 1987:797–801
Effenberger F, Kottmann H (1985) Oxidative phosphonylation of aromatic compounds. Tetrahedron 41:4171–4182
Kagayama T, Nakano A, Sakaguchi S, Ishii Y (2006) Phosphonation of arenes with dialkyl phosphites catalyzed by Mn (II)/Co (II)/O2 redox couple. Org Lett 8:407–409
Mao X, Ma X, Zhang S, Hu H, Zhu C, Cheng Y (2013) Silver-catalyzed highly regioselective phosphonation of arenes bearing electron-withdrawing groups. Eur J Org Chem 20:4245–4248
Ohmori H, Nakai S, Masui M (1979) Anodic oxidation of organophosphorus compounds. Part 2. Formation of dialkyl arylphosphonates via arylation of trialkyl phosphites. J Chem Soc Perkin Trans 1:2023–2026
Nikitin EV, Romakhin AS, Parakin OV, Romanov GV, Kargin YM, Pudovik AN (1983) Electrochemical synthesis of aryl phosphonates. Russ Chem Bull 32:566–569
Cruz H, Gallardo I, Guirado G (2011) Electrochemical synthesis of organophosphorus compounds through nucleophilic aromatic substitution: mechanistic investigations and synthetic scope. Eur J Org Chem 36:7378–7389
Khrizanforov MN, Strekalova SO, Kholin KV, Khrizanforova VV, Kadirov MK, Gryaznova TV, Budnikova YH (2017) Novel approach to metal-induced oxidative phosphorylation of aromatic compounds. Catal Today 279:133–141
Robison CN, Addison JF (1966) Condensation of triethyl phosphonoacetate with aromatic aldehydes. J Org Chem 31:4325–4326
Singh RK, Rogers MD (1985) An efficient synthesis of diethyl coumarin-3-phosphonates. J Heterocycl Chem 22:1713–1714
Bouyssou P, Chenault J (1991) Phosphonates and phosphine oxides as reagents in a one-pot synthesis of coumarins. Tetrahedron Lett 32:5341–5344
Rodios NA, Bojilova A, Terzis A, Raptopoulou CP (1994) Reaction of 3-nitro-and 3-diethylphosphonocoumarin with phenacyl bromide. X-ray molecular structure of 3-nitro-3, 4-phenacylidenecoumarin. J Heterocycl Chem 31:1129–1133
Bojilova A, Nikolova R, Ivanov C, Rodios NA, Terzis A, Raptopoulou CP (1996) A comparative study of the interaction of salicylaldehydes with phosphonoacetates under Knoevenagel reaction conditions. Synthesis of 1, 2-benzoxaphosphorines and their dimers. Tetrahedron 52:12597–12612
Kostka K, Pastuszko S, Kotynski A, Budzisz E (1998) 4-Derivatives coumarin-3-phosphonic acids and esters. Phosphorus Sulfur Silicon Relat Elem 134:199–209
Takeuchi Y, Ueda N, Uesugi K, Abe H, Nishioka H, Harayama T (2003) Convenient synthesis of a simple coumarin from salicylaldehyde and Wittig reagent. IV: improved synthetic method of substituted coumarins. Heterocycles 59:217–224
Zhou P, Jiang YJ, Zou JP, Zhang W (2012) Manganese (III) acetate mediated free-radical phosphonylation of flavones and coumarins. Synthesis 44:1043–1050
Mi X, Huang M, Zhang J, Wang C, Wu Y (2013) Regioselective palladium-catalyzed phosphonation of coumarins with dialkyl H-phosphonates via C–H functionalization. Org Lett 15:6266–6269
Yuan JW, Li YZ, Yang LR, Mai WP, Mao P, Xiao YM, Qu LB (2015) Silver-catalyzed direct Csp2-H radical phosphorylation of coumarins with H-phosphites. Tetrahedron 71:8178–8186
Gao Y, Tang G, Zhao Y (2017) Recent progress toward organophosphorus compounds based on phosphorus-centered radical difunctionalizations, phosphorus, sulfur, and silicon and the related elements. Phosphorus Sulfur Silicon Relat Elem 192(6):589–596
Dudkina YB, Gryaznova TV, Sinyashin OG, Budnikova YH (2015) Ligand-directed electrochemical functionalization of C (sp 2)—H bonds in the presence of the palladium and nickel compounds. Russ Chem Bull 64:1713–1725
Gryaznova T, Dudkina Y, Khrizanforov M, Sinyashin O, Kataeva O, Budnikova Y (2015) Electrochemical properties of diphosphonate-bridged palladacycles and their reactivity in arene phosphonation. J Solid State Electrochem 19:2665–2672
Gryaznova TV, Dudkina YB, Islamov DR, Kataeva ON, Sinyashin OG, Vicic DA, Budnikova Y (2015) Pyridine-directed palladium-catalyzed electrochemical phosphonation of C(sp2)–H bond. J Organomet Chem 785:68–71
Dudkina YB, Gryaznova TV, Kataeva ON, Budnikova YH, Sinyashin OG (2014) Electrochemical CH phosphorylation of 2-phenylpyridine in the presence of palladium salts. Russ Chem Bull 63:2641–2646
Khrizanforov MN, Strekalova SO, Gryaznova TV, Khrizanforova VV, Budnikova YH (2015) New method of metal-induced oxidative phosphorylation of benzene. Russ Chem Bull 64:1926–1932
Jutand A (2008) Contribution of electrochemistry to organometallic catalysis. Chem Rev 108:2300–2347
Budnikova YH (2002) Metal complex catalysis in organic electrosynthesis. Russ Chem Rev 71:111–139
Budnikova YH, Yakhvarov DG, Kargin YM (1997) Arylation and alkylation of white phosphorus in the presence of electrochemically generated nickel (0) complexes. Mendeleev Commun 7:67–68
Budnikova YH, Kargin YM, Perichon J, Nedelec JY (1999) Nickel-catalysed electrochemical coupling between mono-or di-chlorophenylphosphines and aryl or heteroaryl halides. J Organomet Chem 575:63–66
Klein A, Budnikova YH, Sinyashin OG (2007) Electron transfer in organonickel complexes of α-diimines: versatile redox catalysts for C–C or C–P coupling reactions–a review. J Organomet Chem 692:3156–3166
Frontana-Uribe BA, Little RD, Ibanez JG, Palma A, Vasquez-Medrano R (2010) Organic electrosynthesis: a promising green methodology in organic chemistry. Green Chem 12:2099–2119
Yoshida J, Kataoka K, Horcajada R, Nagaki A (2008) Modern strategies in electroorganic synthesis. Chem Rev 108:2265–2299
Fuchigami T, Atobe M, Inagi S (2014) Fundamentals and applications of organic electrochemistry: synthesis, materials, devices. Wiley, Chichester
Milyukov VA, Budnikova YH, Sinyashin OG (2005) Organic chemistry of elemental phosphorus. Russ Chem Rev 74:781–805
Dudkina YB, Khrizanforov MN, Gryaznova TV, Budnikova YH (2014) Prospects of synthetic electrochemistry in the development of new methods of electrocatalytic fluoroalkylation. J Organomet Chem 751:301–305
Dudkina YB, Mikhaylov DY, Gryaznova TV, Tufatullin AI, Kataeva ON, Vicic DA, Budnikova YH (2013) Electrochemical ortho functionalization of 2-phenylpyridine with perfluorocarboxylic acids catalyzed by palladium in higher oxidation states. Organometallics 32:4785–4792
Dudkina YB, Mikhaylov DY, Gryaznova TV, Sinyashin OG, Vicic DA, Budnikova YH (2012) MII/MIII-catalyzed ortho-fluoroalkylation of 2-phenylpyridine. Eur J Org Chem 2012:2114–2117
Khrizanforov M, Gryaznova T, Sinyashin O, Budnikova Y (2012) Aromatic perfluoroalkylation with metal complexes in electrocatalytic conditions. J Organomet Chem 718:101–104
Dudkina YB, Gryaznova TV, Osin YN, Salnikov VV, Davydov NA, Fedorenko SV, Mustafina AR, Vicic DA, Sinyashin OG, Budnikova YH (2015) Nanoheterogeneous catalysis in electrochemically induced olefin perfluoroalkylation. Dalton Trans 44:8833–8838
Khrizanforov M, Strekalova S, Khrizanforova V, Grinenko V, Kholin K, Kadirov M, Burganov T, Gubaidullin A, Gryaznova T, Sinyashin O, Xu L, Vicic DA, Budnikova Y (2015) Iron-catalyzed electrochemical C–H perfluoroalkylation of arenes. Dalton Trans 44:19674–19681
Mikhaylov D, Gryaznova T, Dudkina Y, Khrizanphorov M, Latypov S, Kataeva O, Vicic DA, Sinyashin OG, Budnikova Y (2012) Electrochemical nickel-induced fluoroalkylation: synthetic, structural and mechanistic study. Dalton Trans 41:165–172
Khrizanforov MN, Fedorenko SV, Strekalova SO, Kholin KV, Mustafina AR, Zhilkin MY, Khrizanforova VV, Osin YN, Salnikov VV, Gryaznova TV, Budnikova YH (2016) Ni (iii) complex stabilized by silica nanoparticles as an efficient nanoheterogeneous catalyst for oxidative C–H fluoroalkylation. Dalton Trans 45:11976–11982
Dudkina YB, Kholin KV, Gryaznova TV, Islamov DR, Kataeva ON, Rizvanov IK, Levitskaya AI, Fominykh OD, Balakina MY, Sinyashin OG, Budnikova YH (2017) Redox trends in cyclometalated palladium (II) complexes. Dalton Trans 46:165–177
Mikhaylov DY, Budnikova YH, Gryaznova TV, Krivolapov DV, Litvinov IA, Vicic DA, Sinyashin OG (2009) Electrocatalytic fluoroalkylation of olefins. J Organomet Chem 694:3840–3843
Wei D, Zhu X, Niu JL, Song MP (2016) High-valent-cobalt-catalyzed C–H functionalization based on concerted metalation–deprotonation and single-electron-transfer mechanisms. ChemCatChem 8:1242–1263
Pellissier H, Clavier H (2014) Enantioselective cobalt-catalyzed transformations. Chem Rev 114:2775–2823
Tilly D, Dayaker G, Bachu P (2014) Cobalt mediated C–H bond functionalization: emerging tools for organic synthesis. Catal Sci Technol 4:2756–2777
Cahiez G, Moyeux A (2010) Cobalt-catalyzed cross-coupling reactions. Chem Rev 110:1435–1462
Hess W, Treutwein J, Hilt G (2008) Cobalt-catalysed carbon-carbon bond-formation reactions. Synthesis 22:3537–3562
Byrne FP, Jin S, Paggiola G, Petchey THM, Clark JH, Farmer TJ, Hunt AJ, McElroy CR, Sherwood J (2016) Tools and techniques for solvent selection: green solvent selection guides. Sustain Chem Process 4:1–7
Arends I, Sheldon R, Hanefeld U (2007) Green chemistry and catalysis. Wiley, Weinheim
Kemeling GM (2012) Solvent choices and sustainable chemistry. ChemSusChem 5:2291–2292
Izutsu K (2009) Electrochemistry in nonaqueous solutions, 2nd edn. Wiley, Weinheim
Luca OR, Gustafson JL, Maddox SM, Fenwicka AQ, Smith DC (2015) Catalysis by electrons and holes: formal potential scales and preparative organic electrochemistry. Org Chem Front 2:823–848
Polleux L, Labbé E, Buriez O, Périchon J (2005) CoI- and Co0-bipyridine complexes obtained by reduction of CoBr2bpy: electrochemical behaviour and investigation of their reactions with aromatic halides and vinylic acetates. Chem Eur J 11:4678–4686
Gomes P, Gosmini C, Nédélec J-Y, Périchon J (2000) Cobalt bromide as catalyst in electrochemical addition of aryl halides onto activated olefins. Tetrahedron Lett 41:3385–3388
Budnikova YG, Kafiyatullina AG, Kargin YM, Sinyashin OG (2003) Electrochemical reduction of cobalt and nickel complexes with ligands stabilizing metal in low oxidation state. Russ Chem Bull 52:1504–1511
Budnikova YG, Kafiyatullina AG, Kargin YM, Sinyashin OG (2001) Kinetic regularities of electrochemical reduction of organic halides under the action of cobalt complexes with 2,2′-bipyridine. Russ J Gen Chem 71:231–233
Buettner GR (1987) Spin trapping: ESR parameters of spin adducts 1474 1528V. Free Radical Biol Med 3:259–303
Haire LD, Krygsman PH, Janzen EG, Oehler UM (1988) Correlation of radical structure with EPR spin adduct parameters: utility of the proton, carbon-13, and nitrogen-14 hyperfine splitting constants of aminoxyl adducts of PBN-nitronyl-13C for three-parameter scatter plots. J Org Chem 53:4535–4542
Sheberla D, Tumanskii B, Tomasik AC, Mitra A, Hill NJ, West R, Apeloig Y (2010) Different electronic structure of phosphonyl radical adducts of N-heterocyclic carbenes, silylenes and germylenes: EPR spectroscopic study and DFT calculations. Chem Sci 1:234–241
Tumanskii B, Sheberla D, Molev G, Apeloig Y (2007) Dual character of arduengo carbene–radical adducts: addition versus coordination product. Angew Chem Int Ed 46:7408–7411
Hoffman R (2007) Phosphorus-31 NMR. Hebrew University, Jerusalem
Tang S, Liu Y, Lei A (2018) Electrochemical oxidative cross-coupling with hydrogen evolution: a green and sustainable way for bond formation cell. Chem 4(1):27–45
Acknowledgements
This work was supported by the Russian Science Foundation No. 14-23-00016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khrizanforov, M., Strekalova, S., Khrizanforova, V. et al. Cobalt-Catalyzed Green Cross-Dehydrogenative C(sp2)-H/P-H Coupling Reactions. Top Catal 61, 1949–1956 (2018). https://doi.org/10.1007/s11244-018-1014-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-1014-2