Abstract
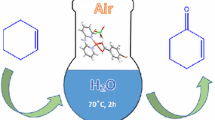
Four new Cu complexes bearing triazolylidene ligands 1-(R)-3-methyl-4-phenyl-1H-1,2,3-triazol-3-ium-5-yl: R = phenyl (2a), mesitylenyl (2b), propyl (2c), hexyl (2d) (NHC) were synthesised in high yields. Characterisation by spectroscopic and analytical methods confirmed the molecular composition of the complexes as NHC–Cu-I. The complexes 2(a–d) bearing NHC wingtip variations were tested as in situ-generated catalysts for homogeneous oxidation catalysis with H2O2 as oxidant. The in situ technique was adopted for ease of application and to circumvent the poor stability of the complexes in solution. The results showed that the NHC–Cu-I complexes are capable of initiating oxidation reactions, yielding ketones/aldehydes as dominant products for the oxidation of alkanes under optimised reaction conditions, with complexes bearing aliphatic N-substituents showing the highest catalytic activities. Oxidation of toluene with 2c resulted in a mixture of benzaldehyde and benzyl alcohol as the main products. Also, 2c catalysed the oxidation of n-octane, yielding a mixture of mainly C-8 oxidation products with over 75% selectivity for the isomeric octanones. Analysis of regioselectivity indicated that the internal \({\text{C}}_{{sp^{3} }}\)–H bonds of n-octane [especially C(2)] are more reactive than the terminal ones.
Graphical abstract





Similar content being viewed by others
References
Peris E, Crabtree RH (2003) C R Chim 6:33–37
Periana RA, Bhalla G, Tenn WJ III, Young KJH, Yang Liu X, Mironov O, Jones CJ, Ziatdinov VR (2004) J Mol Catal A Chem 220:7–25
Díez-González S, Marion N, Nolan SP (2009) Chem Rev 109:3612–3676
Hopkinson MN, Richter C, Schedler M, Glorius F (2014) Nature 510:485–496
Arduengo AJ III, Dias HVR, Harlow RL, Kline M (1992) J Am Chem Soc 114:5530–5534
Fraser PK, Woodward S (2001) Tetrahedron Lett 42:2747–2749
Egbert JD, Cazin CSJ, Nolan SP (2013) Catal Sci Technol 3:912–926
Gao F, McGrath KP, Lee Y, Hoveyda AH (2010) J Am Chem Soc 132:14315–14320
Guzman-Martinez A, Hoveyda AH (2010) J Am Chem Soc 132:10634–10637
Shintani R, Takatsu K, Takeda M, Hayashi T (2011) Angew Chem Int Ed 50:8656–8659
Chun J, Lee HS, Jung G, Lee SW, Kim HJ, Son SU (2010) Organometallics 29:1518–1521
Munz D, Strassner T (2015) Inorg Chem 54:5043–5052
Astakhov AV, Khazipov OV, Degtyareva ES, Khrustalev VN, Chernyshev VM, Ananikov VP (2015) Organometallics 34:5759–5766
Sobkowiak A, Qui A, Liu X, Llobet A, Sawyer DT (1993) J Am Chem Soc 115:609–614
Barton DHR, Doller D, Geletii YV (1991) Mendeleev Commun 1:115–116
Kirillov AM, Kopylovich MN, Kirillova MV, Karabach EY, Haukka M, da Silva MFCG, Pombeiro AJL (2006) Adv Synth Catal 348:159–174
Garcia-Bosch I, Siegler MA (2016) Angew Chem 128:13065–13068
Saravanamurugan S, Palanichamy M, Murugesan V (2004) Appl Catal A 273:143–149
Mac Leod TCO, Kirillova MV, Pombeiro AJL, Schiavon MA, Assis MD (2010) Appl Catal A Gen 372:191–198
Huang G, Luo J, Cai C, Guo Y, Luo G (2008) Catal Commun 9:1882–1885
Mncube SG, Bala MD (2016) J Mol Liq 215:396–401
Soobramoney L, Bala MD, Friedrich HB (2014) Dalton Trans 43:15968–15978
Ramakrishna D, Bhat BR (2011) Inorg Chem Commun 14:690–693
Zhu Q, Lian Y, Thyagarajan S, Rokita SE, Karlin KD, Blough NV (2008) J Am Chem Soc 130:6304–6305
Fernandes RR, Lasri J, Guedes da Silva MFC, da Silva JAL, Fraústo da Silva JJR, Pombeiro AJL (2011) Appl Catal A 402:110–120
Figiel PJ, Kirillov AM, Karabach YY, Kopylovich MN, Pombeiro AJL (2009) J Mol Catal A Chem 305:178–182
Kirillov AM, Kirillova MV, Shul’pina LS, Figiel PJ, Gruenwald KR, Guedes da Silva MFC, Haukka M, Pombeiro AJL, Shul’pin GB (2011) J Mol Catal A Chem 350:26–34
Nasani R, Saha M, Mobin SM, Martins LMDRS, Pombeiro AJL, Kirillovc AM, Mukhopadhyay S (2014) Dalton Trans 43:9944–9954
Kozlov YN, Nizova GV, Shul’pin GB (2005) J Mol Catal A Chem 227:247–253
Shul’pin GB, Drago RS, Gonzalez M (1996) Rus Chem Bull 45:2386–2388
Ciesienski KL, Haas KL, Dickens MG, Tesema YT, Franz KJ (2008) J Am Chem Soc 130:12246–12247
Yacob Z, Shah J, Leistner J, Liebscher J (2008) Synlett 15:2342–2344
Li P, Wang L (2007) Lett Org Chem 4:23–26
Acknowledgements
This project is generously supported by c*change PAR program, the National Research Foundation and the University of KwaZulu-Natal for which we are grateful (Grant No. PAR08).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mncube, S.G., Bala, M.D. Homogeneous oxidation reactions catalysed by in situ-generated triazolylidene copper(I) complexes. Transit Met Chem 44, 145–151 (2019). https://doi.org/10.1007/s11243-018-0278-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0278-5