Abstract
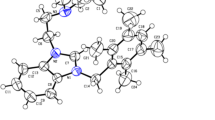
A series of aliphatic nitrile functionalized benzimidazolium salts and their respective mononuclear N-heterocyclic carbene Ag(I)-NHC complexes are reported. The benzimidazolium salts were synthesized by N-alkylation of 1H-benzimidazole with an appropriate alkyl bromide, followed by reaction with either 5-bromovaleronitrile or 6-bromohexanenitrile. The respective mononuclear Ag(I)-NHC complexes were prepared by the reaction of the benzimidazolium salts with Ag2O. All the synthesized compounds were characterized by physico-chemical and spectroscopic techniques. The molecular structures of the two complexes were elucidated through single-crystal X-ray diffraction analyses. Density functional theory was used to model the structures of the other complexes. The benzimidazolium salts and their complexes were screened for cytotoxicity against a breast cancer cell line (MCF-7), using the MTT assay. All the Ag(I)-NHC complexes gave IC50 values ranging from 7.0 ± 1.06 to 12.9 ± 1.55 µM which are comparable to the standard drug, tamoxifen (IC50 = 11.2 ± 1.84 µM), while all of the benzimidazolium salts proved to be inactive.





Similar content being viewed by others
References
Crabtree RH (2014) The Organometallic Chemistry of the Transition Metals, 6th edn, vol 6. Wiley, New York
Ezugwu CI, Kabir NA, Yusubov M, Verpoort F (2016) Coord Chem Rev 307:188–210
Bourissou D, Guerret O, Gabbai FP, Bertrand G (2000) Chem Rev 100:39–91
Nielsen DJ, Cavell KJ, Skelton BW, White AH (2006) Inorg Chim Acta 359:1855–1869
Abu-Surrah AS, Kettunen M (2006) Curr Med Chem 13:1337–1357
Zhao W, Ferro V, Baker MV (2017) Coord Chem Rev 339:1–16
Arduengo AJ, Dias HVR, Calabrese JC, Davidson F (1993) Organometallics 12:3405–3409
Shahini CR, Achar G, Budagumpi S, Tacke M, Patil SA (2017) Appl Organomet Chem 3819:1–15
Kascatan-Nebioglu A, Panzner MJ, Tessier CA, Cannon CL, Youngs WJ (2007) Coord Chem Rev 25:884–895
Youngs WJ, Knapp AR, Wagers PO, Tessier CA (2012) Dalton Trans 41:327–336
Jantke D, Cokoja M, Pothig A, Hermann WA, Kuhn FE (2013) Organometallics 32:41–744
Asif M, Iqbal MA, Hussein MA, Oon CE, Haque RA, Ahamed MBK, Abdul Majid AS, Abdul Majid AMS (2016) Eur J Med Chem 108:177–187
Gautier A, Cisnetti F (2012) Metallomics 4:23–32
Doug AM, Hindi KM, Panzner MJ, Ditto AJ, Yun YH, Youngs WJ (2008) Met-Based Drugs 2008:1–7
Wang CH, Shih WC, Chang HC, Kuo YY, Hung WW, Ong TG, Li WS (2011) J Med Chem 54:5245–5249
Saturnino C, Barone I, Iacopetta D, Mariconda A, Sinicropi MS, Rosano C, Campana A, Catalano S, Longo P, Ando S (2016) Future Med Chem 8:2213–2229
Liu W, Gust R (2016) Coord Chem Rev 329:191–213
Eloy L, Jarrousse AS, Teyssot ML, Gautier A, Morel L, Jolivalt C, Cresteil T, Ronald S (2012) Chem Med Chem 7:805–814
Hickeyners JL, Ruhayel RA, Barnard PJ, Baker MV, Berners-Price SJ, Filipovska A (2008) J Am Chem Soc 130:12570–12571
Sheldrick GM (2015) Acta Crystallogr Sect C 71:3–8
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 Rev B1. Gaussian Inc, Wallingford
Mata JA, Chianese AR, Miecznikowski JR, Poyatos M, Peris E, Faller JW, Crabtree RH (2004) Organometallics 23:1253–1263
Zhong R, Pothig A, Mayer DC, Jandl C, Altmann PJ, Herrmann WA, Kuhn FE (2015) Organometallics 34:2573–2579
Husaini SY, Haque RA, Asekunowo PO, Abdul Majid AMS, Agha MT, Razali MR (2017) J Organomet Chem 840:56–62
Yıgıt B, Gok Y, Ozdemir I, Gunal S (2012) J Coord Chem 65:371–379
Baker MV, Barnarnd PJ, Berners-Prince SJ, Brayshaw SK, Hickey JL, Skelton BW, White AH (2005) J Organomet Chem 690:5625–5635
Ozdemir I, Ozcan EO, Gunal S, Gurbuz N (2010) Molecules 15:2499–2508
Gunal S, Kaloglu N, Ozdemir I, Demir S, Ozedemir I (2012) Inorg Chem Commun 21:142–146
Huynh HV, Yeo CH, Chew YX (2010) Organometallics 29:1479–1486
Khlobystov AN, Blake AJ, Champness NR, Lemenovskii DA, Majouga AG, Zyk NV, Schroder M (2001) Coord Chem Rev 222:155–192
Edward MP, Price DA (2010) Annu Rep Prog Chem 45:381
Asekunowo PO, Haque RA, Razali MR, Avicor SW, Wajidi MFF (2017) Appl Organomet Chem 31:1–15
Siciliano TJ, Deblock MC, Hindi KM, Durmus S, Panzner MJ, Tessier CA, Youngs WJ (2011) J Organomet Chem 696:1066–1071
Zulikha HZ, Haque RA, Budagumpi S, Abdul Majid AMS (2014) Inorg Chim Acta 411:40–47
Patil S, Deally A, Hackenberg F, Kaps L, Muller-Bunz H, Schobert R, Tacke M (2011) Helv Chim Acta 94:1551–1562
Haque RA, Ghdhayeb MZ, Budagumpi S, Salma AW, Ahamed MBK, Abdul Majid AMS (2013) Inorg Chim Acta 394:519–525
Haque RA, Hasanudin N, Iqbal MA, Ahmad A, Hashim S, Abdul Majid AMS, Ahamed MBK (2013) J Coord Chem 66:3211–3228
Fichtner I, Jindrich C, Martin M, Lara CS, Ralf H, Breandan NK, Alison LR, Frauke H, Grainne L, Susan JQ, Lan M, Matthias T (2012) Lett Drug Des Discov 9:815–822
Acknowledgements
R. A. Haque and M. R. Razali thank Universiti Sains Malaysia for RUI Grant (1001/PKIMIA/811346). Sunusi Y. Hussaini thanks Kano University of Science and Technology, Wudil, Nigeria.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hussaini, S.Y., Haque, R.A., Fatima, T. et al. Nitrile functionalized silver(I) N-heterocyclic carbene complexes: DFT calculations and antitumor studies. Transit Met Chem 43, 301–312 (2018). https://doi.org/10.1007/s11243-018-0216-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-0216-6