Abstract
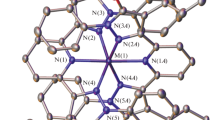
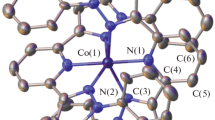
The title complexes \( [{\text{Co}}^{\text{II}}_{2} {\text{L}}^{ 1}_{2} ] \) and \( [{\text{Co}}^{\text{II}} {\text{Co}}^{\text{III}} ( {\text{OAc)L}}^{ 2}_{2} ] \) have been synthesized in excellent yields by reacting Co(OAc)2·4H2O with H2L1 and H2L2, respectively, in acetonitrile solution. Here, [L1]2− and [L2]2− are the deprotonated forms of N,N-bis(2-hydroxybenzyl)-N′,N′-dimethylethylenediamine and N,N-bis(2-hydroxybenzyl)-2-picolylamine, respectively. The crystal structures of \( [{\text{Co}}^{\text{II}}_{2} {\text{L}}^{ 1}_{2} ] \) and \( [{\text{Co}}^{\text{II}} {\text{Co}}^{\text{III}} ( {\text{OAc)L}}^{ 2}_{2} ]{\cdot}{\text{H}}_{ 2} {\text{O}}{\cdot}{\text{CH}}_{ 2} {\text{Cl}}_{ 2} \) were determined by x-ray crystallography. In \( [{\text{Co}}^{\text{II}}_{2} {\text{L}}^{ 1}_{2} ] \), each cobalt atom has distorted trigonal bipyramid geometry, while in \( [{\text{Co}}^{\text{II}} {\text{Co}}^{\text{III}} ( {\text{OAc)L}}^{ 2}_{2} ] \), each cobalt atom has distorted octahedral geometry. Variable temperature magnetic moment measurements show weak antiferromagnetic interaction in \( [{\text{Co}}^{\text{II}}_{2} {\text{L}}^{ 1}_{2} ] \). The magnetic characterization for \( [{\text{Co}}^{\text{II}} {\text{Co}}^{\text{III}} ( {\text{OAc)L}}^{ 2}_{2} ] \) is in agreement with the presence of Co(II) and Co(III) centers.
Graphical Abstract
The title complexes \( [{\text{Co}}^{\text{II}}_{2} {\text{L}}^{ 1}_{2} ] \) and \( \left[ {\left( {{\text{Co}}^{\text{II}} {\text{Co}}^{\text{III}} } \right){\text{L}}^{ 2}_{2} } \right] \) have been synthesized in excellent yields by reacting Co(OAc)2·4H2O with dianionic N2O2 coordinating ligands. In complex 1, each cobalt atom has distorted trigonal bipyramid geometry, while in complex 2, each cobalt atom has distorted octahedral geometry. Variable temperature magnetic moment measurements show weak antiferromagnetic interaction in complex 1. The magnetic characterization for complex 2 is in agreement with the presence of Co(II) and Co(III) centers.







Similar content being viewed by others
References
Ostrovsky SM, Falk K, Pelikan J, Brown DA, Tomkowicz Z, Haase W (2006) Inorg Chem 45:688
Hossain Md J, Yamasaki M, Mikuriya M, Kuribayashi A, Sakiyama H (2002) Inorg Chem 41:4058
Sakiyama H, Ito R, Kumagai H, Inoue K, Sakamoto M, Nishida Y, Yamasaki M (2001) Eur J Inorg Chem (8): 2027
Hernandez-Molina R, Mederos A, Gili P, Dominguez S, Lloret F, Cano J, Julve M, Ruiz-Perez C, Solans X (1997) J Chem Soc Dalton Trans (22): 4327
Fondo M, Ocampo N, Garcıa-Deibe AM, Corbella M, El Fallah MS, Cano J, Sanmartín J, Bermejo MR (2006) J Chem Soc Dalton Trans (41): 4905
Stamatatos TC, Dionyssopoulou S, Efthymiou G, Kyritsis P, Raptopoulou CP, Terzis A, Vicente R, Escuer A, Perlepes SP (2005) Inorg Chem 44:3374
Chiari B, Cinti A, Crispu O, Demartin F, Passini A, Piovesana O (2001) J Chem Soc Dalton Trans (24): 3611
Hemmert C, Gornitzka H, Meunier B (2000) New J Chem 24:949
Chaudhuri P, Querbach J, Wieghardt K, Nuber B, Weiss J (1990) J Chem Soc Dalton Trans (1): 271
Bruker (1998) SMART, SAINT, SADABS, XPREP, SHELXTL, Bruker AXS Inc. Madison. Wisconsin, USA
Sheldrick GM (1994) SHELX TL, version 5.03, Siemens Analytical Instruments, Inc: Madison, WI
Johnson CK (1976) ORTEP. Report ORNL-5138, Oak Ridge National Laboratory. Oak Ridge, TN
Mondal A, Sarkar S, Chopra D, Guru Row TN, Rajak KK (2004) Dalton Trans (20): 3244
West XD, Swearingen KJ, Martinez VJ, Hernandez-Ortega S, El-Sawaf KA, Meurs VF, Castineiras A, Garcia I, Bermejo E (1998) Polyhedron 18:2919
Banerjee S, Chen JT, Lu CZ (2007) Polyhedron 26:686
O’Sullivan C, Murphy G, Murphy B, Hathaway B (1999) J Chem Soc Dalton Trans (11): 1835
Jones MB, MacBeth CE (2007) Inorg Chem 46:8117
Herchel R, Boca R (2005) Dalton Trans (8): 1352
Rodríguez L, Labisbal E, Sousa-Pedrares A, Garcia-Vazquez JA, Romero J, Duran ML, Real JA, Sousa A (2006) Inorg Chem 45:7903
Petit S, Pilet G, Luneau D, Chibotaru LF, Ungur L (2007) Dalton Trans 4582
Liu YH, Tsai HL, Lu YL, Wen YS, Wang JC, Lu KL (2001) Inorg Chem 40:6426
Black D, Blake AJ, Dancey KP, Harrison A, McPartlin M, Parsons S, Tasker PA, Whittaker G, Schröder M (1998) J Chem Soc Dalton Trans (23): 3953
Kahn O (1993) Molecular magnetism. VCH, New York
Acknowledgments
Financial supports from Council of Scientific and Industrial Research, New Delhi, India. And Department of Science and Technology, New Delhi, India. The University Grant Commission, New Delhi is greatly acknowledged. We are also thankful to School of Chemistry, Hyderabad University, India, and Department of Chemistry, Indian Institute of Technology, Guwahati, India for the data collection on the CCD facility setup. The author also acknowledges SAIF, IIT Bombay, India for EPR measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
This file is unfortunately not in the Publisher's archive anymore: Supplementary material 2 (CIF 34 kb)
Rights and permissions
About this article
Cite this article
Singh, R., Banerjee, A., Gordon, Y. et al. Binuclear cobalt(II/II) and cobalt(II/III) chelates with O2N2 ligands: synthesis, structure and magnetic studies. Transition Met Chem 34, 689–694 (2009). https://doi.org/10.1007/s11243-009-9249-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-009-9249-1