Abstract
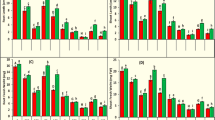
Biomass and in vitro ginsenoside accumulation in cell suspensions of Panax quinquefolius (L.) and P. sikkimensis (Ban.) are differentially affected, under influence of salicylic acid (SA; 100 and 200 µM) and ultrasonic stress (US; 120 W US power, 15 s). SA addition to P. quinquefolius, was observed to lead to decline in biomass accumulation; however SA100 treatment for 5 days led to a 2.6-fold increase in ginsenoside production and Rg3 induction and exudation (6.4 mg/L). Marginally declined growth and ginsenoside productivity was observed on US exposure (% BI or biomass increment = 150.2, ginsenoside = 24.9 mg/L) as compared to unchallenged cultures (% BI = 157.5, ginsenoside = 27.2 mg/L). Co-application of US to SA100 and SA200 treatments for 5 days, although had no significant effect on cell biomass, however led to a further decline in ginsenoside productivity (SA100 + US = 48.6 mg/L, SA200 + US = 27.9 mg/L), when compared to cultures treated only with SA (SA100 = 70.5 mg/L, SA200 = 39.4 mg/L). On the other hand, addition of SA100 and SA200 to P. sikkimensis for 1 week led to a sharp decline in biomass and ginsenoside production, when compared to control cultures. Interestingly, growth and ginsenoside productivity was significantly improved upon co-application of US. US exposure was probably “boosting” mechanism of SA action (SA100 + US = %BI = 124.3, ginsenoside = 57.7 mg/L, SA200 + US = % BI = 135.6, ginsenoside = 102.17 mg/L), when compared to cultures treated with only SA (SA100 = % BI = 96.6, ginsenoside = 19.6 mg/L, SA200 = % BI 103.4, ginsenoside = 36.3 mg/L). In brief, SA100 was the best treatment for maximum ginsenoside productivity specially ginsenoside Rg3 from P. quinquefolius, whereas, SA200 + US was observed to be optimal for P. sikkimensis cell suspensions.



Similar content being viewed by others
Abbreviations
- US:
-
Ultrasonic
- SA:
-
Salicylic acid
- ROS:
-
Reactive oxygen species
- MVA:
-
Mevalonic acid
- IPP:
-
Isopentenyl phosphate
- FPP:
-
Farnesyl diphosphate
- %BI:
-
% Biomass increment
References
Ali MB, Yu KW, Hahn EJ, Paek KY (2006) Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep 25:613–620
Ali MB, Hahn EJ, Paek KY (2007) Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 12:607–621
Baeg IH, So SH (2013) The world ginseng market and the ginseng (Korea). J Ginseng Res 37:1–7
Biswas T, Ajayakumar PV, Mathur AK, Mathur A (2015a) Solvent-based extraction optimisation for efficient ultrasonication-assisted ginsenoside recovery from Panax quinquefolius and P. sikkimensis cell suspension lines. Nat Prod Res 29:1256–1263
Biswas T, Singh M, Mathur AK, Mathur A (2015b) A dual purpose cell line of an Indian congener of ginseng—Panax sikkimensis with distinct ginsenoside and anthocyanin production profiles. Protoplasma 252:697–703
Biswas T, Kalra A, Mathur AK, Lal RK, Singh M, Mathur A (2016) Elicitors’ influenced differential ginsenoside production and exudation into medium with concurrent Rg3/Rh2 panaxadiol induction in Panax quinquefolius cell suspensions. Appl Microbiol Biotechnol 100:4909–4922
Biswas T, Mathur AK, Mathur A (2017) A literature update elucidating production of Panax ginsenosides with a special focus on strategies enriching the anti-neoplastic minor ginsenosides in ginseng preparations. Appl Microbiol Biotechnol 101:4009–4032
Biswas T, Pandey SS, Maji D, Gupta V, Kalra A, Singh M, Mathur A, Mathur AK (2018) Enhanced expression of ginsenoside biosynthetic genes and in vitro ginsenoside production in elicited Panax sikkimensis (Ban.) cell suspensions. Protoplasma. https://doi.org/10.1007/s00709-018-1219-z
Chodisetti B, Rao K, Gandi S, Giri A (2015) Gymnemic acid enhancement in the suspension cultures of Gymnema sylvestre by using the signaling molecules—methyl jasmonate and salicylic acid. In Vitro Cell Dev Biol 51:88–92
Christensen LP (2008) Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res 55:1–99
Gadzovska S, Maury S, Delaunay A, Spasenoski M, Hagège D, Courtois D, Joseph C (2013) The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult 113:25–39
Gurung B, Bhardwaj PK, Rai AK, Sahoo D (2018) Major ginsenoside contents in rhizomes of Panax sokpayensis and Panax bipinnatifidus. Nat Prod Res 32:234–238
Hasan M, Bashir T, Bae H (2017) Use of ultrasonication technology for the increased production of plant secondary metabolites. Molecules 22:1046
Hu X, Neill S, Cai W, Tang Z (2003) Hydrogen peroxide and jasmonic acid mediate oligogalacturonic acid-induced saponin accumulation in suspension-cultured cells of Panax ginseng. Physiol Plant 118:414–421
Huang C, Zhong JJ (2013) Elicitation of ginsenoside biosynthesis in cell cultures of Panax ginseng by vanadate. Process Biochem 48:1227–1234
Huang C, Qian ZG, Zhong JJ (2013) Enhancement of ginsenoside biosynthesis in cell cultures of Panax ginseng by N, N′-dicyclohexylcarbodiimide elicitation. J Biotechnol 165:30–36
Jacques P, Kevers C, Gaspar T, Dommes J, Thonart P (2007) Conditioning Panax vietnamensis cell mass production in bioreactors. Acta Bot Gallica 154:21–26
Kim YS, Hahn EJ, Murthy HN, Paek KY (2004) Adventitious root growth and ginsenoside accumulation in Panax ginseng cultures as affected by methyl jasmonate. Biotechnol Lett 26:1619–1622
Kim OT, Bang KH, Kim YC, Hyun DY, Kim MY, Cha SW (2009) Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tissue Organ Cult 98:25–33
Kim YJ, Zhang D, Yang DC (2015) Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv 33:717–735
Kochan E, Chmiel A (2011) Dynamics of ginsenoside biosynthesis in suspension culture of Panax quinquefolium. Acta Physiol Plant 33:911–915
Kochan E, Krolicka O, Chmiel A (2012) Panax quinquefolium hairy root cultivated in flasks and nutrient sprinkle bioreactor. Acta Physiol Plant 34:1513–1518
Kochkin DV, Kachala VV, Shashkov AS, Chizhov AO, Chirva VY, Nosov AM (2013) Malonyl-ginsenoside content of a cell-suspension culture of Panax japonicus var. repens. Phytochemistry 93:18–26
Lee EJ, Park SY, Paek KY (2015) Enhancement strategies of bioactive compound production in adventitious root cultures of Eleutherococcus koreanum Nakai subjected to methyl jasmonate and salicylic acid elicitation through airlift bioreactors. Plant Cell Tissue Organ Cult 120:1–10
Lin L, Wu J, Ho KP, Qi S (2001) Ultrasound-induced physiological effects and secondary metabolite (saponin) production in Panax ginseng cell cultures. Ultrasound Med Biol 27:1147–1152
Liu J, Wang Q, Sun M, Zhu L, Yang M, Zhao Y (2014) Selection of reference genes for quantitative real-time PCR normalization in Panax ginseng at different stages of growth and in different organs. PLoS ONE 9:e112177
Liu ZB, Chen JG, Yin ZP, Shangguan XC, Peng DY, Lu T, Lin P (2018) Methyl jasmonate and salicylic acid elicitation increase content and yield of chlorogenic acid and its derivatives in Gardenia jasminoides cell suspension cultures. Plant Cell Tissue Organ Cult. https://doi.org/10.1007/s11240-018-1401-1
Mathur A, Mathur AK, Uniyal GC, Pal M, Sangwan RS (2001) Stable high ginsenoside-yielding callus line of Panax quinquefolium (American ginseng) and a method for developing such stable high ginsenoside-yielding callus line. United States Patent US 6326202
Mathur A, Gangwar A, Mathur AK, Sangwan RS, Jain DC (2002) Anthocyanin producing callus line in cultures of Panax sikkimensis and a method of producing Panax sikkimensis line capable of producing anthocyanin. United States Patent US 6368860
Mathur A, Mathur AK, Sangwan RS, Gangwar A, Uniyal GC (2003) Differential morphogenetic responses, ginsenoside metabolism and RAPD patterns of three Panax species. Genet Res Crop Evol 50:245–252
Mehta JK, Haridasan K (1992) The ginsengs in Arunachal Pradesh. Arunachal For News 10:56–58
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult 118:1–6
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev 1:69–79
Phillips DR, Rasbery JM, Bartel B, Matsuda SP (2006) Biosynthetic diversity in plant triterpene cyclization. Curr Opin Plant Biol 9:305–314
Radman R, Saez T, Bucke C, Keshavarz T (2003) Elicitation of plants and microbial cell systems. Biotechnol Appl Biochem 37:91–102
Rahimi S, Kim YJ, Devi BS, Oh JY, Kim SY, Kwon WS, Yang DC (2016) Sodium nitroprusside enhances the elicitation power of methyl jasmonate for ginsenoside production in Panax ginseng roots. Res Chem Intermed 42:2937–2951
Rezaei A, Ghanati F, Dehaghi MA (2011) Stimulation of taxol production by combined salicylic acid elicitation and sonication in Taxus baccata cell culture. Aust J Crop Sci 5:17–24
Russowski D, Maurmann N, Rech SB, Fett-Neto AG (2013) Improved production of bioactive valepotriates in whole-plant liquid cultures of Valeriana glechomifolia. Ind Crops Prod 46:253–257
Sharma SK, Pandit MK (2011) A morphometric analysis and taxonomic study of Panax bipinnatifidus species complex from Sikkim, Himalaya, India. Plant Syst Evol 297(1–2):87–98
Singh RK, Chaudhary BD (1979) Biometrical methods in quantitative genetic analysis. Kalyani Publishers, New Delhi
Sivanandan G, Arun M, Mayavan S, Rajesh M, Jeyaraj M, Dev GK, Manickavasagam M, Selvaraj N, Ganapathi A (2012) Optimization of elicitation conditions with methyl jasmonate and salicylic acid to improve the productivity of withanolides in the adventitious root culture of Withania somnifera (L.) Dunal. Appl Biochem Biotechnol 168:681–696
Smolenskaya IN, Reshetnyak OV, Nosov AV, Zoriniants SE, Chaiko AL, Smirnova YN, Nosov AM (2007) Ginsenoside production, growth and cytogenetic characteristics of sustained Panax japonicus var. repens cell suspension culture. Biol Plant 51:235–241
Taherkhani T, Asghari Zakaria R, Omidi M, Zare N (2017) Effect of ultrasonic waves on crocin and safranal content and expression of their controlling genes in suspension culture of saffron (Crocus sativus L.). Nat Prod Res. https://doi.org/10.1080/14786419.2017.1396598
Tewari RK, Paek KY (2011) Salicylic acid-induced nitric oxide and ROS generation stimulate ginsenoside accumulation in Panax ginseng roots. J Plant Growth Regul 30:396–404
Thanh NT, Murthy HN, Yu KW, Hahn EJ, Paek KY (2005) Methyl jasmonate elicitation enhanced synthesis of ginsenoside by cell suspension cultures of Panax ginseng in 5-l balloon type bubble bioreactors. Appl Microbiol Biotechnol 67:197–201
Thanh NT, Ket NV, Yoeup PK (2007) Effect of medium composition on biomass and ginsenoside production in cell suspension culture of Panax vietnamensis Ha et Grushv. VNU J Sci Nat Sci Technol 23:269–274
Thulke O, Conrath U (1998) Salicylic acid has a dual role in the activation of defence-related genes in parsley. Plant J 14:35–42
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25
Wu J, Ge X (2004) Oxidative burst, jasmonic acid biosynthesis, and taxol production induced by low-energy ultrasound in Taxus chinensis cell suspension cultures. Biotechnol Bioeng 85:714–721
Wu J, Lin L (2001) Elicitor-like effects of low-energy ultrasound on plant (Panax ginseng) cells: induction of plant defense responses and secondary metabolite production. Appl Microbiol Biotechnol 59:51–57
Yu KW, Murthy HN, Jeong CS, Hahn EJ, Paek KY (2005) Organic germanium stimulates the growth of ginseng adventitious roots and ginsenoside production. Process Biochem 40:2959–2961
Yu Y, Zhang WB, Li XY, Piao XC, Jiang J, Lian ML (2016) Pathogenic fungal elicitors enhance ginsenoside biosynthesis of adventitious roots in Panax quinquefolius during bioreactor culture. Ind Crops Prod 294:729–735
Zaheer M, Giri CC (2015) Multiple shoot induction and jasmonic versus salicylic acid driven elicitation for enhanced andrographolide production in Andrographis paniculata. Plant Cell Tissue Organ Cult 122:553–563
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Acknowledgements
The author is grateful to the Director, CSIR-CIMAP, for providing the necessary infrastructure required to complete the study. B. Tanya is also grateful to the University Grants Commission, India for award of a Senior Research Fellowship (SRF) during the course of the present study.
Author information
Authors and Affiliations
Contributions
BT has designed and conducted the experiments and wrote the manuscript. AM has been involved in conceptualizing the experiments and overall supervision and guidance especially with the plant cell cultures. VG has helped with the Real Time PCR experiment set up and analysis. MS has conducted the chemical analysis of the samples. AKM has guided the experimental set up, data analysis and manuscript drafting.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Konstantin V. Kiselev.
Rights and permissions
About this article
Cite this article
Biswas, T., Mathur, A., Gupta, V. et al. Salicylic acid and ultrasonic stress modulated gene expression and ginsenoside production in differentially affected Panax quinquefolius (L.) and Panax sikkimensis (Ban.) cell suspensions. Plant Cell Tiss Organ Cult 136, 575–588 (2019). https://doi.org/10.1007/s11240-018-01538-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-01538-7