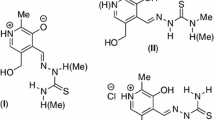
Abstract
The current work reports the synthesis and structural investigation of three novel complexes with 2-acetyl-pyridine-N(4)-phenylthiosemicarbazone (HL1), [Ni(L1)Cl] (1), [Pd(L1)Cl] (2) and [Pt(L1)PPh3]Cl•2MeOH (3). The compounds were structurally characterized by means of single-crystal X-ray crystallography and spectroscopic techniques. All three complexes exhibit tetracoordinated metal centers in a square planar fashion with the thiosemicarbazone acting as a tridentate NNS-donor atoms. Intermolecular hydrogen bonds and π···π stacking interactions, building supramolecular assemblies in the complexes, were analyzed using the Hirshfeld surface. Mass spectrometry data showed the presence in solution of the characteristic fragmentation with the [M+H]+ molecular ion for all complexes. The thermochemical data, estimated by computational chemistry, allowed elucidate the relative intensity of the peaks present in the mass spectrum of the compounds investigated. The antitumor activity and selectivity of the free thiosemicarbazone ligand and their M(II) complexes (M = Ni, Pd, Pt) were evaluated against MCF-7, PBMC, and healthy cells. All compounds studied showed the death of cancer cells observing a great selectivity for the Ni(II) complex.

Graphical abstract









Similar content being viewed by others
References
Donepudi M, Kondapalli K, Amos S et al (2014) Breast cancer statistics and markers. J Cancer Res Ther 10:506–511
Bramati A, Girelli S, Torri V et al (2014) Efficacy of biological agents in metastatic triple-negative breast cancer. Cancer Treat Rev 40:605–613
Esteva FJ, Hubbard-Lucey VM, Tang J et al (2019) Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol 20:e175–e186
Haas BK, Osborne CRC, Vukelja SJ et al (2019) Effect of exercise during adjuvant chemotherapy for breast cancer. J Clin Oncol 37:6524–6524
Lee G, Yang E, Kim S et al (2018) Parapheromones suppress chemotherapy side effects. J Pharmacol Exp Ther 367:215–221
Liu C, Li H, Wang K et al (2019) Identifying the antiproliferative effect of Astragalus polysaccharides on breast cancer: coupling network pharmacology with targetable screening from the Cancer Genome Atlas. Front Oncol 9:1–16
Acharya R, Chacko S, Bose P et al (2019) Structure based multitargeted molecular docking analysis of selected furanocoumarins against breast cancer. Sci Rep 9:15743–15756
Mosquillo MF, Bilbao L, Hernandez F et al (2018) Effect of a new anti-T. cruzi metallic compound based on palladium. Biometals 31:961–974
Bjelosevic H, Guzei IA, Spencer LC et al (2012) Platinum(II) and gold(I) complexes based on 1,1′-bis(diphenylphosphino)metallocene derivatives: synthesis, characterization and biological activity of the gold complexes. J Organomet Chem 720:52–59
Rogolino D, Gatti A, Carcelli M et al (2017) Thiosemicarbazone scaffold for the design of antifungal and antiaflatoxigenic agents: evaluation of ligands and related copper complexes. Sci Rep 7:11214–11226
Palanimuthu D, Poon R, Sahni S et al (2017) A novel class of thiosemicarbazones show multi-functional activity for the treatment of Alzheimer’s disease. Eur J Med Chem 139:612–632
Geersing A, Ségaud N, van der Wijst MGP et al (2018) Importance of metal-ion exchange for the biological activity of coordination complexes of the biomimetic ligand N4Py. Inorg Chem 57:7748–7756
Lopes Ede O, Oliveira CG, Silva PB et al (2016) Novel zinc(II) complexes [Zn(atc-Et)(2)] and [Zn(atc-Ph)(2)]: in vitro and in vivo antiproliferative studies. Int J Mol Sci 17:781–779
Sutradhar M, Barman TR, Rentschler E (2014) Coordination versatility of 1,5-bis(salicylidene)carbohydrazide in Ni(II) complexes. Inorg Chem Commun 39:140–143
Almeida CM, Nascimento GP, Magalhães KG et al (2018) Crystal structures, DNA-binding ability and influence on cellular viability of gold(I) complexes of thiosemicarbazones. J Coord Chem 71:502–519
Beraldo H, Gambino D (2004) The wide pharmacological versatility of semicarbazones, thiosemicarbozones and their metal complexes. Mini-Rev Med Chem 4:31–39
Scarim CB, Jornada DH, Machado MGM et al (2019) Thiazole, thio and semicarbazone derivatives against tropical infective diseases: Chagas disease, human African trypanosomiasis (HAT), leishmaniasis, and malaria. Eur J Med Chem 162:378–395
Shanmugapriya A, Dallemer F, Prabhakaran R (2018) Synthesis, characterisation, crystal structures and biological studies of palladium(ii) complexes containing 5-(2-hydroxy-3-methoxy-phenyl)-2,4-dihydro[1,2,4]triazole-3-thione derivatives. New J Chem 42:18850–18864
de Siqueira LRP, de Moraes Gomes PAT, de Lima Ferreira LP et al (2019) Multi-target compounds acting in cancer progression: focus on thiosemicarbazone, thiazole and thiazolidinone analogues. Eur J Med Chem 170:237–260
Bisceglie F, Pinelli S, Alinovi R et al (2014) Cinnamaldehyde and cuminaldehyde thiosemicarbazones and their copper(II) and nickel(II) complexes: A study to understand their biological activity. J Inorg Biochem 140:111–125
Oliveira CG, Romero-Canelón I, Silva MM et al (2019) Palladium(ii) complexes with thiosemicarbazones derived from pyrene as topoisomerase IB inhibitors. Dalton Trans 48:16509–16517
Liu Y-H, Li A, Shao J et al (2016) Four Cu(ii) complexes based on antitumor chelators: synthesis, structure, DNA binding/damage, HSA interaction and enhanced cytotoxicity. Dalton Trans 45:8036–8049
Sprauten M, Darrah TH, Peterson DR et al (2012) Impact of long-term serum platinum concentrations on neuro- and ototoxicity in cisplatin-treated survivors of testicular cancer. J Clin Oncol 30:300–307
Bermejo E, Castiñeiras A, Domínguez R et al (1999) Preparation, structural characterization, and antifungal activities of complexes of group 12 metals with 2-acetylpyridine- and 2-acetylpyridine-N-oxide-4N-phenylthiosemicarbazones. Z Anorg Allg Chem 625:961–968
SAINT. IMWSa (1999) Area detector control integration software. Bruker Analytical X-ray Instruments, Inc, Karlsruhe
Sheldrick GM (1997) SADABS, program for empirical absorption correction of area detector data. University of Göttingen, Germany, Göttingen
Sheldrick G (2015) SHELXT - integrated space-group and crystal-structure determination. Acta Crystallogr A Found Adv 71:3–8
Sheldrick G (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Struct Chem 71:3–8
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341
Turner MJ MJ, Wolff SK, et al (2017) CrystalExplorer17
McKinnon JJ, Spackman MA, Mitchell AS (2004) Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr Sect B: Struct Sci 60:627–668
Spackman M, Jayatilaka D (2009) Hirshfeld surface analysis. CrystEngComm 11:19–32
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Becke AD (1997) Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals. J Chem Phys 107:8554–8560
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305
Frisch MJ, Trucks GW, Schlegel HB et al (2016) Gaussian 16 Rev. C.01, Wallingford
Honorio-França AC, Hara CCP, Ormonde JVS et al (2013) Human colostrum melatonin exhibits a day-night variation and modulates the activity of colostral phagocytes. J Appl Biomed 11:153–162
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
de Osti R, Fabiana D, Serrano A et al (2012) The in vitro and in vivo antitumour activities of nitrosyl ruthenium amine complexes. Aust J Chem 65:1333–1341
Kovala-Demertzi D, Varagi V, Demertzis MA et al (1996) 2-Acetylpyridinium 4N-Phenylthiosemicarbazone chloride 1.25-hydrate. Acta Crystallogr C Struct Chem 52:2027–2029
Piri Z, Moradi-Shoeili Z, Assoud A (2019) Ultrasonic assisted synthesis, crystallographic, spectroscopic studies and biological activity of three new Zn(II), Co(II) and Ni(II) thiosemicarbazone complexes as precursors for nano-metal oxides. Inorg Chim Acta 484:338–346
PIdS M, Graminha A, Pavan FR et al (2010) Palladium(II) complexes with thiosemicarbazones: syntheses, characterization and cytotoxicity against breast cancer cells and Anti-Mycobacterium tuberculosis activity. J Braz Chem Soc 21:1177–1186
Okuniewski A, Rosiak D, Chojnacki J et al (2015) Coordination polymers and molecular structures among complexes of mercury(II) halides with selected 1-benzoylthioureas. Polyhedron 90:47–57
Indoria S, Lobana TS, Singh D et al (2015) Stabilization of CuII–I bonds using 2-benzoylpyridine thiosemicarbazones – synthesis, structure, spectroscopy, fluorescence, and cyclic voltammetry. Eur J Inorg Chem 2015:5106–5117
Ferraz KSO, Da Silva JG, Costa FM et al (2013) N(4)-Tolyl-2-acetylpyridine thiosemicarbazones and their platinum(II,IV) and gold(III) complexes: cytotoxicity against human glioma cells and studies on the mode of action. BioMetals 26:677–691
Rebolledo AP, Vieites M, Gambino D et al (2005) Palladium(II) complexes of 2-benzoylpyridine-derived thiosemicarbazones: spectral characterization, structural studies and cytotoxic activity. J Inorg Biochem 99:698–706
Suni V, Kurup MRP, Nethaji M (2007) Structural and spectral investigations on some new Ni(II) complexes of di-2-pyridyl ketone N(4)-phenylthiosemicarbazone. Polyhedron 26:3097–3102
Arion VB, Rapta P, Telser J et al (2011) Syntheses, electronic structures, and EPR/UV−Vis−NIR spectroelectrochemistry of nickel(II), copper(II), and zinc(II) complexes with a Tetradentate ligand based on S-methylisothiosemicarbazide. Inorg Chem 50:2918–2931
Ervin KM (2001) Experimental techniques in gas-phase ion thermochemistry. Chem Rev 101:391–444
Zeglis BM, Divilov V, Lewis JS (2011) Role of Metalation in the topoisomerase IIα inhibition and antiproliferation activity of a series of α-heterocyclic-N4-substituted thiosemicarbazones and their Cu(II) complexes. J Med Chem 54:2391–2398
Dobrova A, Platzer S, Bacher F et al (2016) Structure–antiproliferative activity studies on l-proline- and homoproline-4-N-pyrrolidine-3-thiosemicarbazone hybrids and their nickel(ii), palladium(ii) and copper(ii) complexes. Dalton Trans 45:13427–13439
Funding
This study is financially supported by the FAPDF (process number 0193.001545/2017), UnB, FINEP/CTINFRA and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. RLTP and RPO are financially supported by the São Paulo Research Foundation (FAPESP), grants 2011/07623–8 and 2017/24856–2. RLTP is supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (313648/2018–2) through a research fellowship.
Author information
Authors and Affiliations
Contributions
The manuscript was written with contributions from all authors. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Crystallographic data for the structures in this work have been deposited to the Cambridge Crystallographic Data Centre, CCDC 1893403-1893404. Copies of the available material can be obtained free of charge by application to the Director, CCDC, 12 Union Road, Cambridge CH2 1EZ, UK (Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk). Crystallographic data are in CIF files.
ESM 1
(DOCX 2559 kb)
ESM 2
(CIF 421 kb)
ESM 3
(CIF 398 kb)
ESM 4
(CIF 686 kb)
Rights and permissions
About this article
Cite this article
Almeida, C.M., de Carvalho, J.G.M., Fujimori, M. et al. Structural investigation of group 10 metal complexes with thiosemicarbazone: crystal structure, mass spectrometry, Hirshfeld surface and in vitro antitumor activity. Struct Chem 31, 2093–2103 (2020). https://doi.org/10.1007/s11224-020-01564-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01564-2