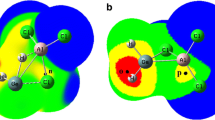
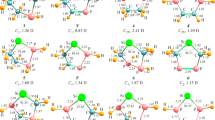
Abstract
The structures of phosphinidene germylenoid HP=GeLiF were studied for the first time by using DFT (density functional theory) calculations. The geometries were optimized at the B3LYP/6-311+G (d, p) level at first and then the single-point energies were calculated at QCISD/6-311++G (d, p) level. Theoretical calculations predicted that HP=GeLiF has two equilibrium structures, the p-complex (1) and the three-membered-ring (2) structures, in which structure 1 has the lower energy and is more stable than 2. To exploit the reactivity of HP=GeLiF, the cycloaddition reaction of 1 and ethylene was investigated at the same level of theory. From the potential energy profile, we predicted that the cycloaddition reaction has one dominant reaction pathway. The calculated result shows that the dominant reaction pathway is a [2 + 2] cycloaddition reaction which is the interaction of two π bonds in HP=GeLiF and ethylene molecule, and a four-membered-ring P-heterocyclic germylene is formed. Since sp3 hybridization of Ge atom in this four-membered-ring germylene, it may further react with another ethylene and finally forming a spiro-Ge-heterocyclic compound involving phosphorus. This means that this reaction involves a [2 + 2] cycloaddition as the initial step, and then a [2 + 1] cycloaddition carried out.




Similar content being viewed by others
References
Harper WW, Ferrall EA, Hilliard RK, Stogner SM, Grev RS, Clouthier DJ (1997). J Am Chem Soc 119:8361–8362
Boone AJ, Magers DH, Leszczyński J (1998). Int J Quantum Chem 70:925–932
Stogner SM, Grev RS (1998). J Chem Phys 108:5458–5464
Hostutler DA, Smith TC, Li H, Clouthier DJ (1999). J Chem Phys 111:950–958
Liao HY, Su MD, Chu SY (2000). Inorg Chem 39:3522–3525
Firme CL, Antunes OAC, Esteves PM (2009). Chem Phys Lett 468:129–133
Lu XH, Han JF, Xu YH, Shi LY, Lian ZX (2010). Russ J Phys Chem A 84:980–986
Hostutler DA, Clouthier DJ, Pauls SW (2002). J Chem Phys 116:1417–1423
He SG, Tackett BS, Clouthier DJ (2004). J Chem Phys 121:257–264
Lee VY, Sekiguchi A (2011). Inorg Chem 50:12303–12314
Hao Q, Lu TX, Wilke JJ, Simmonett AC, Yamaguchi Y, Fang DC, Schaefer HF (2012). J Phys Chem A 116:4578–4589
Lu XH, Lian ZX, Li YQ, Wang ZN (2012). Struct Chem 23:809–813
Lu XH, Lian ZX, Li YQ, Wang ZN (2012). Struct Chem 23:1769–1775
Lu XH, Lian ZX, Li YQ, Wang ZN (2012). J Mol Model 18(9):4209–4215
Lu XH, Lian ZX, Li YQ, Wang ZN (2012). Russ J Phys Chem A 86:1869–1874
Lu XH, Lian ZX, Liu DT, Bao WJ (2013). Int J Quantum Chem 113:1562–1567
Lu XH, Li YQ, Bao WJ, Liu DT (2013). Comput Theor Chem 1007:76–81
Lu XH, Li YQ, Liu DT, Bao WJ (2015). Res Chem Intermed 41:235–243
Shoda S, Iwata S, Yajima K, Yagi K, Ohnishi Y, Kobayashi S (1997). Tetrahedron 53:15281–15295
Iwamoto T, Masuda H, Ishida S, Kabuto C, Kira M (2004). J Organomet Chem 689:1337–1341
Kassaee MZ, Ghambarian M, Musavi SM (2005). J Organomet Chem 690:4692–4703
Filippou AC, Stumpf KW, Chernov O, Schnakenburg G (2012). Organometallics 31:748–755
Li WZ, Yang FX, Cheng JB, Li QZ, Gong BA (2012). Chin J Struct Chem 31:19–26
Tan XJ, Wang WH, Li P, Wang QF, Zheng GX, Liu F (2008). J Organomet Chem 693:475–482
Tan XJ, Wang WH, Li P, Liu F (2009). Russ J Phys Chem A 83:1355–1362
Li WZ, Cheng JB, Gong BA, Xiao CP (2006). J Organomet Chem 691:5984–5987
Li WZ, Tan HN, Xiao CP, Gong BA, Cheng JB (2007). Acta Phys -Chim Sin 23:1811–1814
Satge J, Couret C, Escudié J (1971). J Organomet Chem 30:c70–c74
Couret C, Satge J, Andriamizaka JD, Escudie J (1978). J Organomet Chem 157:c35–c39
Escudie J, Couret C, Andrianarison M, Satge J (1987). J Am Chem Soc 109:386–390
Andrianarison M, Couret C, Declercq JP, Dubourg A, Escudie J, Ranaivonjatovo H, Satge J (1988). Organometallics 7:1545–1548
Ranaivonjatovo H, Escudié J, Couret C, Satgé J (1991). J Organomet Chem 415:327–333
Ranaivonjatovo H, Escudié J, Couret C, Declercq JP, Dubourg A, Satgé J (1993). Organometallics 12:1674–1681
Kandrirodi A, Declercq JP, Dubourg A, Ranaivonjatovo H, Escudie J (1995). Organometallics 14:1954–1960
Rodi AK, Ranaivonjatovo H, Escudié J, Kerbal A (1997). Phosphorus Sulfur 126:157–169
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li H, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr PJE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.01. Gaussian, Inc., Wallingford
Becke AD (1993). J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988). Phys Rev B 37:785–789
Hehre WJ, Radom L, Schleyer PR, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Gauss J, Cremer D (1988). Chem Phys Lett 150:280–286
Salter EA, Trucks GW, Bartlett RJ (1989). J Chem Phys 90:1752–1766
Gonzalez C, Schlegel HB (1991). J Chem Phys 95:5853–5860
Funding
This work was supported by the National Natural Science Foundation Committee of China (No. 21103145), the Natural Science Foundation of Shandong Province (No. ZR2016BM23), and the Special Foundation of Youth Academic Backbone of Yantai University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 49 kb)
Rights and permissions
About this article
Cite this article
Yan, B., Yuan, T., Li, W. et al. Structures of the phosphinidene germylenoid HP=GeLiF and its cycloaddition reaction with ethylene. Struct Chem 29, 1647–1653 (2018). https://doi.org/10.1007/s11224-018-1142-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1142-0