Abstract
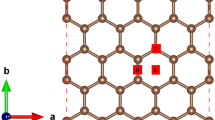
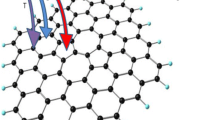
The investigation of methane adsorption on surface of graphene is of high interest as graphene can be tuned as required, also the favorable adsorption properties may be employed to create cost-effective novel storage devices. In the present study, we investigate the adsorption characteristics of methane (CH4) on eight different kinds of hydrogen-capped graphene sheet with variable and high number of carbon atoms using density functional theory methods. Our results infer that the methane adsorption is high on defected graphene than that of pristine and less charge transfer between CH4 and graphene. The physisorbed methane molecule on graphene sheet has enhanced adsorption energy with a high number of carbon atoms and the value is −0.184 and −0.185 eV (pristine graphene) and −0.188 and −0.191 eV (defected graphene). Due to high barrier, the transfer of electrons from graphene to methane is hard rather than methane to graphene. Further, it is found that the energy gap value of the hydrogen-capped graphene sheets decreases upon adsorption of methane. The reduced density gradient (RDG) scatter graph shows the interaction to be weak van der Waals interaction with the steric repulsion in the graphene sheet.












Similar content being viewed by others
References
Baxter J, Bian Z, Chen G, Danielson D, Dresselhaus MS, Fedorov AG, Fisher TS, Jones CW, Maginn E, Kortshagen U, Manthiram A, Nozik A, Rolison DR, Sands T, Shi L, Wu Y (2009) Energy Environ 2:559–588
Morris RE, Wheatley PS (2008) Angew Chem Int Ed 47:4966–4981
Saha D, Bao Z, Jia F, Deng S (2010) Environ Sci Technol 44:1820–1826
Mendoza-cortes JL, Pascal TA, Goddard WA (2011) J Phys Chem A 115:13852–13857
Zhou L, Liu X, Sun Y, Li J, Zhou Y (2005) J Phys Chem B 109:22710–22714
Kowalczyk P, Brualla L, Andrzej Z, Bhatia SK (2007) J Phys Chem C 111:5250–5257
Wang S (2007) Energy Fuel 21:953–956
Lee J, Balathanigaimani MS, Kang H, Shim W, Kim C, Moon H (2007) J Chem Eng Data 52:66–70
Morales-cas AM, Moya C, Coto B, Vega LF, Calleja G (2007) J Phys Chem C 111:6473–6480
Gallo M, Glossman-mitnik D (2009) J Phys Chem C 113:6634–6642
Chakraborty SN, Gelb LD (2012) J Phys Chem B 116:2183–2197
Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, Dubonos SV, Firsov AA (2005) Nature 438:197–200
Booth TJ, Blake P, Nair RR, Jiang D, Hill EW, Bangert U, Bleloch A, Gass M, Novoselov KS, Katsnelson MI, Geim AK (2008) Nano Lett 8:2442–2446
Thierfelder C, Witte M, Blankenburg S, Rauls E, Schmidt WG (2011) Surf Sci 605:746–749
Kim HK, Chan MHW (1984) Phys Rev Lett 53:170–173
Brenner K, Yang Y, Murali R (2011) Carbon 50:637–645
Thrower PA (1969) In: Walker PL (ed) Chemistry and physics of carbon. Dekker, New York
Stone AJ, Wales DJ (1986) Chem Phys Lett 128:501–503
Dinadayalane TC, Leszczynski J (2007) Chem Phys Lett 434:86–91
Dinadayalane TC, Murray JS, Concha MC, Politzer P, Leszczynski J (2010) J Chem Theory Comput 6:1351–1357
Motahari S, Shayeganfar F, Neek-amal M (2012) Solid State Commun 152:225–230
Reddy CD, Ramasubramaniam A, Shenoy VB, Zhang YW (2009) Appl Phys Lett 94:101904
Sun C, Boutilier MSH, Au H, Poesio P, Bai B, Karnik R, Hadjiconstantinou NG (2014) Langmuir 30:675–682
Jiang D, Cooper VR, Dai S (2009) Nano Lett 9:4019–4024
Zhang YH, Chen YB, Zhou KG, Liu CH, Zeng J, Zhang HL, Peng Y (2009) Nanotechnology 20:185504
Boukhvalov DW, Katsnelson MI (2008) Nano Lett 8:4374–4379
Hassani A, Taghi M, Mosavian H, Ahmadpour A, Farhadian N (2016) Comput Theor Chem 1084:43–50
Denis PA (2008) Chem Phys 353:79–86
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta Jr JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi Pomelli RC, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Gaussian, Inc, Wallingford
Tachikawa H, Iyama T (2014) Solid State Sci 28:41–46
Umadevi D, Sastry GN (2014) Curr Sci 106:1224–1234
Lu T Multiwfn 2.1, http://multiwfn.codeplex.com/
Skowron ST, Lebedeva IV, Popov AM, Bichoutskaia E (2015) Chem Soc Rev 44:3143–3176
Zhu Y, Su H, Jing Y, Guo J, Tang J (2016) Appl Surf Sci 387:379–384
Carrillo I, Rangel E, Magan LF (2009) Carbon 7:2758–2760
Qiu N, Xue Y, Guo Y, Sun W, Chu W (2012) Comput Theor Chem 992:37–47
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang WT (2010) J Am Chem Soc 132:6498–6506
Humphrey W, Dalke A, Schulten K (1996) J Mol Graph Model 14:33–38
Kose E, Karabacak M, Bardak F, Atac A (2016) J Mol Struct 1123:284–299
Li D, Yang Y, Li C, Liu Y (2017) J Lumin 182:15–21
Chithiraikumar S, Gandhimathi S, Neelakantan MA (2017) J Mol Struct 1137:569–580
Son Y, Cohen ML, Louie SG (2006) Phys Rev Lett 97:216803
Acknowledgments
One of the authors (SV) highly acknowledges the Department of Science and Technology (DST-SERB), Government of India, for the financial support in the form of a project under Grant SR/FTP/PS-115/2011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Increase in number of carbon atoms and incorporation of defects increases the affinity of methane adsorption on graphene sheet.
• Physisorption of methane molecule prefers to be adsorbed above the carbon atom of the surface of graphene rather than the center of the six-, five-, or seven-membered ring.
• The energy gap of the methane-adsorbed graphene sheet is lower than the bare graphene.
Rights and permissions
About this article
Cite this article
Anithaa, V.S., Shankar, R. & Vijayakumar, S. DFT-based investigation on adsorption of methane on pristine and defected graphene. Struct Chem 28, 1935–1952 (2017). https://doi.org/10.1007/s11224-017-0988-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-0988-x