Abstract
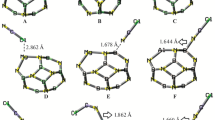
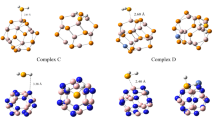
The adsorption behavior and electronic sensitivity of the pristine, and Al-doped B24N24 (Al-BN) nanoclusters toward two carbonyl halides (F2CO, and Cl2CO (phosgene)) were investigated using density functional calculations. It was found that the carbonyl halides interact with the pristine cluster very weakly while by replacing a B atom of the cluster with an Al atom, the adsorption energy is significantly increased. The electronic properties of the pristine BN nanocluster are not sensitive to F2CO but after the Cl2CO adsorption, its HOMO-LUMO gap (E g) is significantly decreased. Despite this decrease, the BN cage is still insulator and cannot be used as a sensor. The Al-doping overcomes this problem by a primarily reduction in the E g (from 6.45 to 4.88 eV). Our calculations indicate that by F2CO and Cl2CO adsorptions, the Al-BN cluster is transformed from insulator to a semiconductor. This phenomenon largely increases the electrical conductivity of Al-BN and can produce electrical noise which is responsible for the gas detection. Also, it is shown that the Al-BN selectively responses to F2CO and Cl2CO gases.






Similar content being viewed by others
References
Eckert WG (1991) Mass deaths by gas or chemical poisoning: a historical perspective. The American journal of forensic medicine and pathology 12(2):119–125
Crouzel C, Hinnen F, Maitre E (1995) Radiosynthesis of methyl and heptyl [11 C] Isocyanates from [11 C] phosgene, application to the synthesis of carbamates:[11 C] physostygmine and [11 C] heptylphysostigmine. Appl Radiat Isot 46(3):167–170
Borak J, Diller WF (2001) Phosgene exposure: mechanisms of injury and treatment strategies. J Occup Environ Med 43(2):110–119
Fawcett F, Tullock C, Coffman D (1962) The chemistry of carbonyl fluoride. I. The fluorination of organic compounds. J Am Chem Soc 84(22):4275–4285
Hill HH, Martin SJ (2002) Conventional analytical methods for chemical warfare agents. Pure Appl Chem 74(12):2281–2291
Rinsland C, Zander R, Brown L, Farmer C, Park J, Norton R, Russell J, Raper O (1986) Detection of carbonyl fluoride in the stratosphere. Geophys Res Lett 13(8):769–772
Bogue R (2008) Nanosensors: a review of recent progress. Sens Rev 28(1):12–17
Beheshtian J, Peyghan AA, Bagheri Z, Kamfiroozi M (2012) Interaction of small molecules (NO, H2, N2, and CH4) with BN nanocluster surface. Struct Chem 23(5):1567–1572
Vessally E, Soleimani-Amiri S, Hosseinian A, Edjlal L, Bekhradnia A (2017) The Hartree-Fock exchange effect on the CO adsorption by the boron nitride nanocage. Physica E 87(3):308–311
Peyghan AA, Baei MT, Moghimi M, Hashemian S (2012) Adsorption and electronic structure study of imidazole on (6, 0) zigzag single-walled boron nitride nanotube. J Clust Sci 24:31–47
Beheshtian J, Peyghan AA, Bagheri Z (2013) Sensing behavior of Al-rich AlN nanotube toward hydrogen cyanide. J Mol Model 19(6):2197–2203
Beheshtian J, Peyghan AA, Bagheri Z, Tabar MB (2014) Density-functional calculations of HCN adsorption on the pristine and Si-doped graphynes. Struct Chem 25(1):1–7
Peyghan AA, Rastegar SF, Hadipour NL (2014) DFT study of NH3 adsorption on pristine, Ni-and Si-doped graphynes. Phys Lett A 378(30):2184–2190
Yuan L, Hu M, Wei Y, Ma W (2016) Enhanced NO2 sensing characteristics of Au modified porous silicon/thorn-sphere-like tungsten oxide composites. Appl Surf Sci 389:824–834
Golberg D, Bando Y, Tang C, Zhi C (2007) Boron nitride nanotubes. Adv Mater 19(18):2413–2432
Vessally E, Soleimani-Amiri S, Hosseinian A, Edjlali L, Bekhradnia A (2017) A comparative computational study on the BN ring doped nanographenes. Appl Surf Sci 396(2):740–745
Nejati K, Hosseinian A, Edjlali L, Vessally E (2017) The effect of structural curvature on the cell voltage of BN nanotube based Na-ion batteries. J Mol Liq 229(3):167–171
Nejati K, Hosseinian A, Bekhradnia A, Vessally E, Edjlal L (2017) Na-ion batteries based on the inorganic BN nanocluster anodes: DFT studies. J Mol Graph Model 74(6):1-7
Safari L, Vessally E, Bekhradnia A, Hosseinian A, Edjlali L (2017) A DFT study on the sensitivity of two-dimensional BN nanosheet to nerve agents cyclosarin and tabun. Thin Solid Films 623(2):57-163
Deng ZY, Zhang JM, Xu KW (2016) Adsorption of SO2 molecule on doped (8, 0) boron nitride nanotube: a first-principles study. Phys E 76:47–51
Srivastava P, Jaiswal NK, Sharma V (2014) First-principles investigation of armchair boron nitride nanoribbons for sensing PH3 gas molecules. Superlattice Microst 73:350–358
Boshra A, Jadidi S, Goudarzi M, Niroumand S, Mohammadi M (2012) DFT study of endohedral atoms effect on electrophilicity of B16N16 boron nitride nanocage: comparative analyses. J Clust Sci 23(2):297–310
Srivastava P, Sharma V, Jaiswal NK (2015) Adsorption of COCl2 gas molecule on armchair boron nitride nanoribbons for nanosensor applications. Microelectron Eng 146:62–67
Bai L, Zhou Z (2007) Computational study of B-or N-doped single-walled carbon nanotubes as NH3 and NO2 sensors. Carbon 45:2105–2110
Hp Z, Luo X, Lin X, Yp Z, Tang Pp LX, Tang Y (2015) Band structure of graphene modulated by Ti or N dopants and applications in gas sensoring. J Mol Graph Model 61:224–230
Wang R, Zhu R, Zhang D (2008) Adsorption of formaldehyde molecule on the pristine and silicon-doped boron nitride nanotubes. Chem Phys Lett 467:131–135
Beheshtian J, Peyghan AA, Noei M (2013) Sensing behavior of Al and Si doped BC3 graphenes to formaldehyde. Sensors Actuators B Chem 181:829–834
Ahmadi Peyghan A, Omidvar A, Hadipour NL, Bagheri Z, Kamfiroozi M (2012) Can aluminum nitride nanotubes detect the toxic NH3 molecules? Phys E 44:1357–1360
Pannopard P, Khongpracha P, Probst M, Limtrakul J (2009) Gas sensing properties of platinum derivatives of single-walled carbon nanotubes: a DFT analysis. J Mol Graph Model 28:62–66
Ahmadi Peyghan A, Hadipour NL, Bagheri Z (2013) Effects of Al doping and double-antisite defect on the adsorption of HCN on a BC2N nanotube: density functional theory studies. J Phys Chem C 117:2427–2432
Chen C, Xu K, Ji X, Miao L, Jiang J (2014) Enhanced adsorption of acidic gases (CO2, NO2 and SO2) on light metal decorated graphene oxide. Phys Chem Chem Phys 16:11031–11036
Feng D, Zhang Y, Shi W, Li X, Ma H (2010) A simple and sensitive method for visual detection of phosgene based on the aggregation of gold nanoparticles. Chem Commun 46(48):9203–9205
Beheshtian J, Peyghan AA, Bagheri Z (2012) Detection of phosgene by Sc-doped BN nanotubes: a DFT study. Sens Actuators B: Chem 171:846–852
Virji S, Kojima R, Fowler JD, Villanueva JG, Kaner RB, Weiller BH (2009) Polyaniline nanofiber composites with amines: novel materials for phosgene detection. Nano Res 2(2):135–142
Oku T, Nishiwaki A, Narita I, Gonda M (2003) Formation and structure of B24N24 clusters. Chem Phys Lett 380:620–623
Ma Z, Zhang Y, Li F, Chen H (2016) Comparative study of H2 adsorption on B24 N24, Al24N24 and B12Al12N24 clusters. Comput Mater Sci 117:71–75
Koi N, Oku T, Suaki Suganuma K (2005) Effects of endohedral element in B24N24 clusters on hydrogenation studied by molecular orbital calculations. Phys E 29:541–545
Rouzbehani GM, Boshra A, Seif A (2009) B24N24 nanocages: a GIAO density functional theory study of 14N and 11B nuclear magnetic shielding and electric field gradient tensors. Monatshefte für Chemie-Chemical Monthly 140:255–263
Mileev M, Kuzmin S, Parfenyuk V (2006) Ab initio calculations of structure and stability of small boron nitride clusters. J Struct Chem 47:1016–1021
Weinhold F, Landis CR (2001) Natural bond orbitals and extensions of localized bonding concepts. Chemistry Education Research and Practice 2:91–104
Becke AD (1993) Density-functional thermochemistry. III The role of exact exchange The Journal of chemical physics 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785
Grimme S (2004) Accurate description of van der Waals complexes by density functional theory including empirical corrections. J Comput Chem 25:1463–1473
Tabtimsai C, Ruangpornvisuti V, Wanno B (2013) Density functional theory investigation of the VIIIB transition metal atoms deposited on (5,5) single-walled carbon nanotubes. Phys E 49:61–671
Mishra AK (2015) DFT study of structural, vibrational and electronic properties of polyaniline pernigraniline model compounds. Journal of Computational Science 10:195–208
Abdulsattar MA (2011) SiGe superlattice nanocrystal pure and doped with substitutional phosphorus single atom: density functional theory study. Superlattice Microst 50(4):377–385
Tomic S, Montanari B, Harrison NM (2008) The group III–V’s semiconductor energy gaps predicted using the B3LYP hybrid functional. Phys E 40:2125–2127
Samadizadeh M, Rastegar SF, Peyghan AA (2015) The electronic response of nano-sized tube of BeO to CO molecule: a density functional study. Struct Chem 26(3):809–814
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
O’boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845
Boys SF, Fd B (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Hadipour NL, Ahmadi Peyghan A, Soleymanabadi H (2015) Theoretical study on the Al-doped ZnO nanoclusters for CO chemical sensors. J Phys Chem C 119(11):6398–6404
Grüning M, Marini A, Rubio A (2006) Density functionals from many-body perturbation theory: the band gap for semiconductors and insulators. J Chem Phys 124(15):154108–154115
Strehlow W, Cook E (1973) Compilation of energy band gaps in elemental and binary compound semiconductors and insulators. J Phys Chem Ref Data 2(1):163–200
Hybertsen MS, Louie SG (1986) Electron correlation in semiconductors and insulators: band gaps and quasiparticle energies. Phys Rev B 34(8):5390–5399
Peyghan AA, Noei M, Yourdkhani S (2013) Al-doped graphene-like BN nanosheet as a sensor for para-nitrophenol: DFT study. Superlattice Microst 59:115–122
Soltani A, Raz SG, Rezaei VJ, Dehno Khalaji A, Savar M (2012) Ab initio investigation of Al- and Ga-doped single-walled boron nitride nanotubes as ammonia sensor. Appl Surf Sci 263:619–625
Shao P, Kuang X-Y, Ding L-P, Yang J, Zhong M-M (2013) Can CO 2 molecule adsorb effectively on Al-doped boron nitride single walled nanotube? Appl Surf Sci 285:350–356
Behmagham F, Vessally E, Massoumi B, Hosseinian A, Edjlali LA Computational study on the SO2 adsorption by the pristine, Al, and Si doped BN nanosheets. Superlattice Microst. doi:10.1016/j.spmi.2016.09.040
Acknowledgements
The authors gratefully acknowledge the financial support of this work by the Mazandaran University of Medical Sciences ‘‘Professor’s Projects Funds’’.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nejati, K., Hosseinian, A., Vessally, E. et al. A theoretical study on the electronic sensitivity of the pristine and Al-doped B24N24 nanoclusters to F2CO and Cl2CO gases. Struct Chem 28, 1919–1926 (2017). https://doi.org/10.1007/s11224-017-0977-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-0977-0