Abstract
It is shown that the H ⋯ B contacts in 1-silacyclohex-2-enes are clearly stabilizing and strong, whereas those in 1-silacyclopent-2-enes are much weaker. This result is supported by analysis of QTAIM-based parameters and appropriate structural changes taking place upon the open form → closed form transformation and is in full agreement with previous NMR spectroscopic data [Wrackmeyer et al. (2006) Appl Organometal Chem 20:99–105]. Also, the influence of electronic and steric effects originating from the presence of specific substituents on the strength of the H ⋯ B contacts is discussed in detail. Some problems and ideas associated with the use of the so-called open-closed method utilized in assessing values of interaction energies are discussed in detail. Particular attention is paid to the correct choice of reference open systems. It is shown that their partial geometry optimization leads to reliable values of interaction energies.
Similar content being viewed by others
Introduction
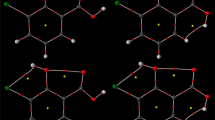
Wrackmeyer is most likely the first who paid attention to the presence and the bonding character of a Si–H–B bridge in organoboron compounds [1–4]. The role of this bridge in the formation of heterocyclic systems containing both silicon and boron atoms was discussed on the basis of both NMR and IR spectroscopic data [1]. Also, the presence of a Si–H–B bridge in 3-bora-4-methylene-homoadamantane was determined on the basis of X-ray structural measurements [2]. More importantly for the present article, based on the NMR spectra obtained for two members (1 and 2 in Fig. 1) of 1-silacyclopent-2-enes, Wrackmeyer et al. [4] have concluded that Si–H–B bridges in these molecules are either absent or extremely weak. This was to be in opposition to their 1-silacyclohex-2-ene counterparts (3 and 4 in Fig. 1), where existences of Si–H–B bridges have been confirmed by similar spectroscopic data [4]. This difference in Si–H–B bonding effect was attributed to stronger repulsion between silyl groups in both 1-silacyclohex-2-enes forcing the exocyclic relevant silyl group to approach closer to the 9-borabicyclo[3.3.1]nonane group (9-BBN) and, as a consequence, considerably reducing the H ⋯ B distance.
Structural formulas of 1-silacyclopent-2-enes and 1-silacyclohex-2-enes investigated by Wrackmeyer et al. [4]
Bonding properties of the Si–H ⋯ Y bridge are well known [5–9]. For example, in case of Y being a transition metal, this interaction is called an agostic bond [10–13] or a σ interaction [12–17] depending on a specific situation, whereas if Y is an electron-deficient element (as, for example, boron), this type of interaction was called a “charge-inverted hydrogen bond” (CIHB) [11–13, 18–25]. Therefore, the electron-deficient Si–H–B bridge (this term was being used by Wrackmeyer et al. [1–4]) in investigated systems in fact represents one of the examples of intramolecular charge-inverted hydrogen bonds (IMCIHB).
Taking the above into account, it is really a tempting challenge to assess strengths of H ⋯ B interactions in 3 and 4, and particularly in 1 and 2. It can be done by computing values of the interaction energy of H ⋯ B. Although this energetical parameter is not accessible to experimental measurements, it is relatively easily accessible to theoretical methods of molecular modeling. As widely believed, the interaction energy directly describes the strength of a (local) intramolecular interaction. Taking into account the NMR spectroscopic data obtained by Wrackmeyer et al. [4], one should expect clearly negative, i.e. showing stabilizing character of H ⋯ B, values of interaction energies obtained for both 1-silacyclohex-2-enes, i.e. 3 and 4, whereas for both 1-silacyclopent-2-enes (1 and 2), they should be either positive or only slightly negative. In the former case, our results would suggest repulsive character of the H ⋯ B contact, whereas in the latter, this contact would be weakly bonding. In addition to these four molecules investigated earlier by Wrackmeyer et al. [4] (Fig. 1), we also have performed similar computations for their simplified derivatives (see the ’Investigated systems’ section). This approach allows us to discuss influence of both electronic and steric effects originating from the presence of specific substituents on the strength of investigated Si–H ⋯ B bridges.
The other purpose of this article is to shed light on some problems relating to the definition of the interaction energy (of an intramolecular H ⋯ Y contact) itself. In particular, an importance of the choice of a reasonable reference open system will be discussed in detail, showing that this is not so easily-done task as it might at first seem. In our opinion, this issue is not raised often enough [19, 26–31].
Computational methodology
Geometry optimizations and frequency calculations to analyze characters of obtained stationary points have been performed using Gaussian 09 program [32]. Lack of imaginary frequencies has confirmed that obtained geometries correspond to local minima on the potential energy hypersurface. For some reference systems, also, partial geometry optimizations have been performed as indicated in the text. Analysis of the topology of the electron density distribution has been made by means of quantum theory of atoms in molecules (QTAIM) [33–35] using the AIMAll package [36]. All calculations have been performed utilizing the PBE0 [37–39] exchange-correlation functional of density functional theory and the 6-311G(d,p) basis set [40, 41]. It was shown that PBE0 gives reasonable molecular structures [12, 42] as well as electronic densities [43, 44]. On the other hand, the 6-311G(d,p) basis set is of valence triple zeta quality and, additionally, includes polarization functions for all atoms.
Energy of an intramolecular interaction
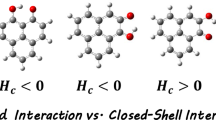
In contrast to the energy of an intermolecular interaction (e.g. a hydrogen bond), the energy of an intramolecular interaction is not a definable quantity due to the fact that any attempt of extracting the energy of the interaction of interest from the total energy of a system hosting this interaction introduces high degree of discretion for the choice of a reference system [27–31]. Nevertheless, in spite of this, some additive schemes leading to numbers understood as interaction energies have been proposed. Among them, the so-called open-closed method [45, 46] seems to be both the most popular [26–29, 45–58] and straightforward. According to this method, the interaction energy is just a difference between total energies of the closed form of a molecule, i.e. the form hosting the relevant interaction, and the open one obtained by a proper rotation of either the donor or the acceptor group and, as a consequence, characterized by a cleavage of the relevant interaction (see Fig. 2).
It is understood that in general different interaction energy values are obtained as a result of different reference open systems used [28].
What is more, having one of these open systems already chosen, one encounters a new question, namely whether or not the geometry of the open system should be fully optimized [28, 58]. Unfortunately, only in a small piece of articles where the open-closed method is utilized, this question is addressed [19, 28, 29, 45, 46, 58]. Most likely, Schuster was the first who suggested [45] to use the open form having ’the least changes in molecular geometry besides a cleavage of the H-bond‘ pointing out that the open reference system ’need not be a local minimum of the energy surface‘ [46]. In his opinion, performing single-point calculations for the open form ’seems to be more appropriate‘ than the full geometry optimization of the open reference form ’in cases where it can be applied, because it does not mix a large energy of isomerization into the H-bond energy‘ [46]. Schuster underlined, however, that any splitting of the change in total energy into pure isomerization and H-bond energy is artificial in fact [46]. As already mentioned, acceptance of this approach leads to single-point energy calculations for the open form, for which all geometry parameters (but a rotation angle of course) are taken from the closed form and then kept constant (i.e. frozen). This situation is depicted on the left hand side of Fig. 3, where E c, \(E_{\mathrm {o}}^{\text {f,c}}\) and \(E_{\mathrm {c}}^{\mathrm {f}}\) mean total energies of closed, fictitious open and fictitious closed forms, respectively. It should be noted that this approach is in line with the definition of the intermolecular interaction energy, ΔE int = E(AB) − E(A) − E(B), where individual monomers A and B have geometries from the complex AB.
On the contrary, the other approach, which nowadays seems to be a standard procedure [57], in the open-closed method arises if the full geometry optimization (rather than single-point calculations) of the open form is performed (leading to E o; Fig. 3). This approach takes into account the fact that the geometry (structure) change that takes place upon the open form → closed form transformation is a consequence of the relevant interaction. In other words, the geometry of the interaction-hosting system is marked by the presence of this interaction. In the picture of intermolecular interactions, this approach corresponds to fully isolated monomers A and B possessing their own geometries. In this case, ΔE int would include deformation energies as well. More precisely, both these ΔE int values in fact correspond to different energy parameters—interaction and binding energies, respectively. Similarly, in the case of intramolecular interactions, both approaches introduce in fact different definitions of the interaction energy—parameter that—to remind—is in fact not strictly defined by quantum chemistry [29–31]. Both these definitions of the (intramolecular) interaction energy correspond to different partition schemes of the total energy of the closed form as shown in Fig. 3. Of course, \(|E_{\mathrm {o}}| > |E_{\mathrm {o}}^{\text {f,c}}|\) and hence, one should observe that \({\Delta } E_{\text {int}}^{\text {OPT}} < {\Delta } E_{\text {int}}^{\text {SP}}\).
At this point, one encounters a new problem. Quite often reference open systems have new important interactions (either attractive or repulsive) [27–31, 51–53]. As a consequence, one obtains either under- (\({\Delta } E_{\text {int,a}}^{\text {OPT}}\)) or overestimated (\({\Delta } E_{\text {int,r}}^{\text {OPT}}\)) values of interaction energies, respectively, as shown in Fig. 4. Some examples will be discussed later.
Moreover, in many instances, the structure of the fully optimized open form may also be considerably different than that of the closed form. This case can be manifested by significantly different values of bond lengths and of plane or dihedral angles. For example, Buemi et al. [28] rebuked the use of the most extended enol and enethiol tautomers of thiomalondialdehyde as reference structures [27] since the trans configuration of double bonds seems to be too different than the cis one in the closed form. It is easy to imagine other examples. The amino group is usually somewhat pyramidal, whereas it becomes flat in closed form featuring conjugated system of double bonds as, e.g. in 3-aminoprop-2-enal. Boryl−BH2 group may be perpendicular (or nearly so) to the molecular chain in closed form, whereas, on the contrary, it may be in plane in the open one. It will be shown that the latter happens if the 9-BNN group is substituted by -BH2. It is seen from Fig. 4 that too attractive interaction(s) in the open reference form or its significantly extended structure can even lead to changed sign of the interaction energy [19].
Taking the above into account, we proposed [19] performing partial (i.e. constrained) geometry optimization in such cases, where one freezes only those geometric parameters whose optimization would lead to either some new important interactions or to considerably different structures. Although in most cases, it is sufficiently to freeze one or two dihedral angles that determine positions of donor and acceptor groups, sometimes, it is necessary to freeze other geometrical parameters as well. Of course, the full geometry optimization of the open form may still be performed if only it will not lead to any new important interactions or considerable structural changes in at least some parts of the molecule. The idea of performing constrained geometry optimization of the open reference form [19] is also utilized in our estimates of interaction energies of H ⋯ B.
Investigated systems
Initial calculations have been performed for 9-(2-(dimethylsilyl)-1,1-diphenyl-1-silacyclopenta-2-en-3-yl)-9-borabicyclo(3.3.1)nonane (1 in Fig. 1) utilizing its experimental structure determined from X-ray crystalographic measurements [4] and available via Cambridge Structural Database [59] as MEDSUK (1A \(_{\exp }\)). Also, its analogue with −SiMe2H rotated about the Si–C bond has been used as a reference molecule (1a \(_{\exp }\)). Their fully optimized analogues are labelled as 1A and 1a, respectively. Since experimental structures of 2, 3 and 4 have not been determined, fully optimized geometries of both open and closed forms of these molecules have been obtained (2A, 3A, 4A and 2a, 3a, 4a, respectively). Although used for computing ν SiH frequency shifts, 1a, 2a, 3a and 4a have, however, been found as being not fully correct references for interaction energies. Instead, reference open systems obtained after partial (see further text) geometry optimizations have been used (symbols with prime). Eventually, similar calculations for some derivatives of all these systems have also been performed. Letter ’B’ (or ’b’) has been used if the −SiMe2H group has been substituted by −SiH3, ’C’ (or ’c’) if 9−BBN has been substituted by −BMe2, and ’D’ (or ’d’) if 9−BBN has been substituted by −BH2 (see Fig. 5).
Similar as for 1A, 2A, 3A and 4A, partially rather than fully optimized open forms have been used for estimations of interaction energies of H ⋯ B contacts in the closed forms. For the reasoning of their use, see the ’Energy of an intramolecular interaction’ section and discussion of results. Structures of fully optimized forms of all investigated systems as well as their open forms used as references for the interaction energy estimates are shown in Fig. 6.
Results and discussion
Discussed values of most important geometric parameters relating to the HSiCCB quasi-ring in open and closed forms of investigated systems are gathered in Table 1.
Local deformations of structure are determined by parameters P, \(\theta _{\text {HSiCC}}^{\text {def}}\), \(\theta _{\text {HSiCSi}}^{\text {def}}\) and θ SiCCB, where P, that can be called as pyramidalization parameter [25] of the trigonal boryl subunit, is just a difference between the round angle and the sum of all E1-B-E2 (where E1 and E2 is C or H) angles in this subunit, \(\theta _{\text {HSiCC}}^{\text {def}}\) and \(\theta _{\text {HSiCSi}}^{\text {def}}\) are deformation angles showing positional deviation of the H atom from the plane (for closed forms: \(\theta _{\text {HSiCC}}^{\text {def}} = \theta _{\text {HSiCC}}\) and \(\theta _{\text {HSiCSi}}^{\text {def}} = 180^{\circ } - \theta _{\text {HSiCSi}}\), for open forms: \(\theta _{\text {HSiCC}}^{\text {def}} = 180^{\circ } - |\theta _{\text {HSiCC}}|\) and \(\theta _{\text {HSiCSi}}^{\text {def}} = |\theta _{\text {HSiCSi}}|\)), and θ SiCCB is just the Si–C–C–B dihedral angle. Since in all primed systems, the θ HSiCC angle has been taken as 180° and then kept constant, the value of 0° for \(\theta _{\text {HSiCC}}^{\text {def}}\) for all these systems is a direct consequence of partial geometry optimization.
General features of closed forms
We begin analysis of our results shown in Table 1 on changes taking place upon the open form → closed form transformation, i.e when the Si–H ⋯ B bridge is formed. It is clearly seen that formation of this bridge leads to shorter Si ⋯B distances and longer Si–H bonds. Elongations of Si–H are accompanied by red-shifts of ν SiH. Moreover, more significant pyramidalization of the boryl fragment is observed as well. All these effects are geometrical evidences of bonding character of H ⋯ B contacts in the closed forms. This result is then confirmed by negative values of interaction energies. Only in case of nB (n = 1–4) systems the bonding character of H ⋯ B may be uncertain if one takes into account that the error of the open-closed method may be of the order of some 1–2 kcal/mol. Clearly, however, the bonding character of the SiH ⋯ B bridge is considerably weakened if the −SiMe2H group is substituted by the −SiH3 one. Conversely, compared to −SiH3, the −SiMe2H group leads to stronger H ⋯ B interaction. This most likely is due to the electron-donating properties of the methyl group.
1-Silacyclopent-2-enes vs 1-silacyclohex-2-enes
The main purpose of this article is to assess the strength of Si–H ⋯ B bridges in 1-silacyclopent-2-enes and to compare them to those obtained for similar 1-silacyclohex-2-enes (Fig. 1). As already mentioned in the ’Introduction’ section, on the basis of NMR spectroscopic data, Wrackmeyer et al. [4] have concluded that in the former group, the Si–H ⋯ B bridges are either absent or extremely weak, whereas, in the latter, Si–H ⋯ B bridges have been said to exist.
Our estimation of the interaction energy of the Si–H ⋯ B bridge in 1A \(_{\exp }\) gave −6.6 kcal/mol—quite large value considering, e.g. relatively long H ⋯ B distance (2.647 Å), acute α SiHB angle (103.4°) and relatively high value of ν SiH (2083 cm−1) comparing to 3A \(_{\exp }\) (1919 cm−1) where Si–H ⋯ B was said to exist [4]. So high (absolute) value results, however, from experimental values of geometric parameters that do not correspond to fully optimized ones. Considerably lower values of d Si⋯B (3.224 Å), d H⋯B (2.647 Å) and d SiH (1.325 Å) in 1A \(_{\exp }\) comparing to similar values in fully optimized 1A (3.276, 2.712 and 1.504 Å, respectively; Table 1) suggest significant packing forces in the former. Importantly, the full geometry optimization of 1A \(_{\exp }\) leads to modest lowering of its total energy than of 1a \(_{\exp }\). As a consequence, the energy of the H ⋯ B contact in the fully optimized 1A is much lower, amounting to −2.6 kcal/mol only. This value is even lower in 2A and amounts to −1.7 kcal/mol. Thus, if the H ⋯ B contacts in 1A and 2A are binding, they are indeed very weak as noted by Wrackmeyer et al. [4]. At this point, it is noteworthy that similar estimates for 3A and 4A have given energies of −5.1 and −3.9 kcal/mol, respectively (Table 1). Thus, the stabilizing character of the H ⋯ B contacts in both these systems is rather doubtless. This conclusion is in line with earlier spectroscopic results and the fact that, as we have shown in Fig. 7, the H ⋯ B interactions in both these systems are traced by corresponding bond paths, whereas similar bond paths are absent in case of the former two molecules, i.e. 1A and 2A.
As Bader has stated, the presence of a bond path (on a molecular graph) and a concomitant bond critical point indicate stabilizing interaction [33, 34]. On the other hand, however, a presumably binding interaction is not necessarily traced by a bond path [60, 61]. As already discussed in the earlier subsection, apart from negative values of interaction energies, changes of geometrical parameters also indicate stabilizing character of H ⋯ B contacts in 1A and 2A.
Electronic and steric effects
We have already shown that either clearly or presumably binding character of H ⋯ B contacts in 3A and 4A or 1A and 2A, respectively, results from highly electrodonor properties of methyl groups present in −SiMe2H. If they are substituted by hydrogens then the H ⋯ B contacts become hardly binding (ΔE int amounts to ca. −0.5 kcal/mol only for all systems nB; n = 1-4). On the contrary, Si ⋯B and H ⋯ B distances become much longer and the Si–H bond becomes much shorter. Also, the α SiHB angle becomes more acute (and close to the right angle) indicating that Si–H and C–B bonds are almost parallel to each other (see also Fig. 6). Another evidence of much weaker interactions in nB comparing to nA are higher values of ν SiH in the former. All these effects are particularly evident in respective pairs of 1-silacyclohex-2-enes.
It should be mentioned that the bulky 9-BBN group in nA and nB is not completely advantageous in our estimations of ΔE int in these systems because of possible H ⋯H repulsions in their na’ and nb’ open forms (Fig. 6). For this reason, we suspect that values of ΔE int obtained for these systems may be somewhat overestimated (Fig. 4). Nevertheless, both qualitative results as well as relations among computed values should be proper since the same structural changes, and thus, interatomic interactions occur in all pairs of open and closed forms. Moreover, all (9-BBN)H ⋯H(SiMe2H) or (9-BBN)H ⋯H(SiH3) distances are longer than ca. 2.15 or 2.42 Å found in 4a’ and 4b’, respectively, suggesting that repulsions are rather negligible as being no larger than ca. 0.5 kcal/mol (MP2/aug-cc-pVTZ) [31].
To eliminate the bulky 9-BBN group, we have considered nC systems possessing the -BMe2 group in place of 9-BBN (Fig. 5). Although this small group should reduce H ⋯H repulsions in open nc’ forms (the shortest H ⋯H distances are ca. 2.4 Å in 1c’ and 4c’ and ca. 2.5 Å in 2c’ and 3c’), on the other hand, it should lead to weaker Si–H ⋯ B bridges due to certain electron density shift from both methyl groups of −BMe2 to the formally empty 2p orbital on B. Indeed, in the case of both 1-silacyclohex-2-enes, the interaction energy decreases from −5.1 kcal/mol in 3A to −4.1 kcal/mol in 3C and from −3.9 kcal/mol in 4A to −3.0 kcal/mol in 4C (Table 1). Weakening of the relevant H ⋯ B interactions is also reflected in lower values of electron density at bond critical points of these interactions (ρ b). They amount to 0.063 and 0.055 au in 3A and 3C, respectively, whereas 0.060 and 0.053 au in 4A and 4C, respectively. In addition to the electron density, also, the delocalization index [62, 63] of H and B atoms (which is a measure of the number of electron pairs shared by these two atomic basins), DI(H,B), decreases as expected (from 0.206 au in 3A to 0.183 au in 3C and from 0.198 au in 4A to 0.175 au in 4C). Larger values of both ρ b and DI(H,B) in the former pair of molecules also indicate that H ⋯ B interactions should be stronger in 3A and 3C than in 4A and 4C. This suggestion is in line with our estimates of ΔE int. Similar decreases of ΔE int are, however, not observed for both 1-silacyclopent-2-enes, for which ΔE int is either the same (1A → 1C; ΔE int = −2.6 kcal/mol) or slightly increases (from −1.7 kcal/mol in 2A to −2.1 kcal/mol in 2C). This indicates that our estimates of ΔE int are more reliable for 1-silacyclohex-2-enes than those for 1-silacyclopent-2-enes and may most likely result from considerably higher values of ΔE int for the former group of systems.
In turn, to eliminate the electron donating properties of methyl groups in −BMe2, we have also investigated systems nD (and nd’) possessing −BH2 in place of −BMe2 (Fig. 5). Moreover, this substitution further reduces H ⋯H interactions in open reference forms. On the other hand, however, the −BH2 group is prone to rotate around the C–B bond (going in plane) so as to possibly form dihydrogen bond and to conjugate with the C=C bond of the ring. For this reason, the open reference nd’ systems have been obtained by freezing not only θ HSiCC (at 180°) but θ HBCC as well (at ±90°). Thus, −BH2 in nd’ was kept perpendicularly to the BCC plane. Since the nD systems feature rather significant pyramidalization of the −BH2 fragment (P amounts to ca. 10° in 1D and 2D and ca. 11° in 3D and 4D; Table 1), the pyramidalization energy, i.e. the energy that is needed to deform flat −BH2 group in nd’ to its somewhat pyramidal shape in nD, is included in our estimates of ΔE int. As discussed in the ’Energy of an intramolecular interaction’ section, this and other (small) deformations are, however, consequences of the H ⋯ B interactions in the nD closed forms. As expected, one obtains considerably high values of ΔE int. In 1D and 2D 1-silacyclopent-2-enes, they have been estimated as ca. −10 kcal/mol, whereas in both 1-silacyclohex-2-enes, ΔE int are higher amounting to −13.8 kcal/mol in 3D and −12.6 kcal/mol in 4D. So high values of ΔE int are in line with relatively large values of ρ b (ca. 0.08 au for all nD systems). It is noteworthy that among all 1L and 2L (L = A-D) only 1D and 2D feature a H ⋯ B bond path. Moreover, also, DI(H,B) values are rather high (0.291 au for 1D and 2D and 0.297 au for 3D and 4D). The characteristic feature of these systems is that d SiH > d H⋯B (Table 1), indicating a highly advanced transfer of H toward B. Similar effect has also been reported [18] in H3SiH ⋯BH3 (better written as H3Si ⋯H2⋅BH2). Interestingly, reported binding energy of this dimer amounts to -11.7 kcal/mol (MP2/aug-cc-pVTZ) [18]—closely to our estimates for nD.
Conclusions
The main purpose of this article is to assess strengths of H ⋯ B contacts in some 1-silacyclopent-2-enes and 1-silacyclohex-2-enes investigated earlier by Wrackmeyer et al. [4]. For this purpose, we have computed interaction energies of these contacts utilizing so-called open-closed method. In full agreement with previous conclusions based on NMR spectroscopic data [4], we have shown that the H ⋯ B contacts in investigated 1-silacyclohex-2-enes are indeed strong, whereas those in 1-silacyclopent-2-enes are much weaker. This result is supported by appropriate changes of geometrical parameters that take place upon the open form → closed form transformation and by reported values of some QTAIM-based parameters.
Influence of both the electronic and steric effects originating from the presence of specific substituents on the strength of relevant H ⋯ B contacts is discussed in detail. We have shown that relatively strong H ⋯ B interactions found in both 1-silacyclopent-2-enes and 1-silacyclohex-2-enes result from the presence of two methyl groups in −SiMe2H. If they are substituted by hydrogens, then H ⋯ B interactions become hardly bonding. On the other hand, if the 9-BBN group is subsituted by −BH2, then the investigated Si–H ⋯ B bridges feature many of the characteristic effects attesting to their considerable strength as also shown by high values of interaction energies and QTAIM-based parameters.
Some problems and ideas associated with the use of the open-closed method are discussed in detail. In particular, we have paid attention to the correct choice of the reference open system. It has been shown that utilization of the partial geometry optimization of the reference open system leads to reliable results.
References
Wrackmeyer B, Tok O L, Bubnov YN (1999) The first Si−H−B bridge: combination of 1,1-organoboration and hydrosilylation. Angew Chem Int Ed 38:124–126
Wrackmeyer B, Milius W, Tok O L (2003) Reaction of alkyn-1-yl(diorganyl)silanes with 1-boraadamantane: Si−H−B bridges confirmed by the molecular structure in the solid state and in solution. Chem Eur J 9:4732–4738
Wrackmeyer B, Tok OL, Khan A, Badshah A (2005) 1,1-Organoboration of silylethynyltin compounds studied by multinuclear magnetic resonance spectroscopy: isomerization at the C=C bonds and electron-decient Si-H-B bridges. Appl Organometal Chem 19:1249–1256
Wrackmeyer B, Tok OL, Milius W, Khan A, Badshah A (2006) 1-Silacyclopent-2-enes and 1-silacyclohex-2-enes bearing functionally substituted silyl groups in 2-positions. Novel electron-deficient Si-H-B bridges. Appl Organometal Chem 20:99–105
Schubert U (1990) η 2 Coordination of Si-H σ Bonds to Transition Metals. Adv Organomet Chem 30:151–187
Kubas GJ (2001) Metal Dihydrogen and σ-Bond Complexes. Kluwer Academic/Plenum Publisher, New York
Clot E, Eisenstein O (2004) Agostic Interactions from a Computational Perspective: One Name, Many Interpretations. Struct Bonding (Berlin) 113:1–36
Lin Z (2002) Structural and bonding characteristics in transition metal-silane complexes. Chem Soc Rev 31:239–245
Lein M (2009) Characterization of Agostic Interactions in Theory and Computation. Coord Chem Rev 253:625–634
Schneider JJ (1996) Si−H, C−H Activation by transition metal complexes: a step towards isolable alkane complexes Angew Chem Int Ed Engl 35:1068–1075
Jabłoński M (2015) QTAIM-Based Comparison of Agostic Bonds and Intramolecular Charge-Inverted Hydrogen Bonds. J Phys Chem A 119:4993–5008
Jabłoński M (2015) Geometry- and QTAIM-Based Comparison of Intramolecular Charge-Inverted Hydrogen Bonds, M ⋯(H−Si) ”Agostic Bond”, and M ⋯(η 2-SiH) σ Interactions. J Phys Chem A 119:11384–11396
Jabłoński M (2016) Comparative study of geometric and QTAIM-based differences between X-H ⋯ Y intramolecular charge-inverted hydrogen bonds, M1⋯(H-X) agostic bonds and M2⋯(η 2-XH) σ interactions (X = Si, Ge; Y = Al, Ga; M1 = Ti, Co; M2 = Mn, Fe, Cr). Comput Theor Chem 1096:54–65
Choi SH, Feng J, Lin Z (2000) Understanding the Readiness of Silane Dissociation in Transition Metal η 2-Silane Complexes Cp(CO)2M[ η 2-H(SiH3n Cl n )] (M = Mn, Tc, and Re; n = 1 − 3). Organometallics 19:2051–2054
McGrady GS, Sirsch P, Chatterton N, Ostermann A, Gatti C, Altmannshofer S, Herz V, Eickerling G, Scherer W (2009) Nature of the Bonding in Metal-Silane σ-Complexes. Inorg Chem 48:1588–1598
Jabłoński M (2016) Systematic studies of the influence of electronegative X (X = F and Cl) substituents on the structure of the Mn-H-Si unit and the strength of the Mn ⋯(η 2-SiH) σ interaction in Cp(OC)2Mn[ η 2-H(SiH3−n X n )] (n = 0−3) complexes. Comput Theor Chem 1076:51–56
Jabłoński M (2016) Conciliatory Inductive Model Explaining the Origin of Changes in the η 2-SiH Bond Length Caused by Presence of Strongly Electronegative Atoms X (X = F, Cl) in Cp(OC)2Mn[ η 2-H(SiH3−n X n )] (n = 0 − 3) Complexes. J Phys Chem A 120:4211–4222
Jabłoński M (2009) Binding of X-H to the lone-pair vacancy: Charge-inverted hydrogen bond. Chem Phys Lett 477:374–376
Jabłoński M (2010) Full vs. constrain geometry optimization in the open-closed method in estimating the energy of intramolecular charge-inverted hydrogen bonds. Chem Phys 376:76–83
Jabłoński M (2010) Intramolecular charge-inverted hydrogen bond. J Mol Struct: THEOCHEM 948:21–24
Jabłoński M (2012) Theoretical insight into the nature of the intermolecular charge-inverted hydrogen bond. Comput Theor Chem 998:39–45
Jabłoński M, Sokalski WA (2012) Physical nature of interactions in charge-inverted hydrogen bonds. Chem Phys Lett 552:156–161
Jabłoński M (2014) Charge-inverted hydrogen bond vs. other interactions possessing a hydridic hydrogen atom. Chem Phys 433:76–84
Udagawa T, Tachikawa M (2015) H/D Isotope Effect on Charge-Inverted Hydrogen-Bonded Systems: Systematic classification of three different types in H3XH⋯YH3 (X = C, Si, or Ge, and Y = B, Al, or Ga) with Multicomponent Calculation. J Comput Chem 36:1647–1654
Yourdkhani S, Jabłoński M (2017) Revealing the Physical Nature and the Strength of Charge-Inverted Hydrogen Bonds by SAPT(DFT), MP2, SCS-MP2, MP2C, and CCSD(T) Methods, J Comput Chem 38:773–780
Buemi G, Grabowski SJ (eds) (2006) Hydrogen Bonding—New insights, vol 3. Springer, Dordrecht, The Netherlands
Craw JS, Bacskay GB (1992) Quantum-chemical Studies of Hydrogen Bonding involving Thioxoketones, Thienols, Thioformaldehyde and Hydrogen Sulfide with Specific Reference to the Strength of Intramolecular Hydrogen Bonds. J Chem Soc Faraday Trans 88:2315–2321
Buemi G, Zuccarello F (2002) Is the intramolecular hydrogen bond energy valuable from internal rotation barriers J Mol Struct (Theochem) 581:71–85
Jabłoński M, Kaczmarek A, Sadlej AJ (2006) Estimates of the Energy of Intramolecular Hydrogen Bonds. J Phys Chem A 110:10890–10898
Jabłoński M (2012) Energetic and Geometrical Evidence of Nonbonding Character of Some Intramolecular Halogen ⋯Oxygen and Other Y ⋯Y Interactions. J Phys Chem A 116:3753–3764
Jabłoński M, Monaco G (2013) Different Zeroes of Interaction Energies As the Cause of Opposite Results on the Stabilizing Nature of C-H ⋯O Intramolecular Interactions. J Chem Inf Model 53:1661–1675
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta Jr JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ Gaussian 09, Gaussian, Inc., Wallingfor
Bader RFW (1990) Atoms in Molecules: A Quantum Theory. Oxford University Press, New York
Popelier PLA (2000) Atoms in Molecules. An Introduction. Longman, Singapore
Matta CF, Boyd RJ (2007) The Quantum Theory of Atoms in Molecules. Wiley-VCH, Weinheim
Keith TA (2015) AIMAll (Version 15.05.18), TK Gristmill Software, Overland Park KS, USA, <aim.tkgristmill.com>
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Perdew JP, Burke K, Ernzerhof M (1997) Errata: generalized gradient approximation made simple. Phys Rev Lett 78:1396
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158–6170
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72(1980):650-654
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11-18. J Chem Phys 72:5639–5648
Zyder M, Kochel A, Handzlik J, Szymanska-Buzar T (2009) Photochemical reaction of Mo(CO)6 with Et2GeH2: NMR and DFT studies of reaction products; crystal structure of a novel complex [{Mo(μ- η 2-H–GeEt2)(CO)4}2]. Organometallics 28:5857–5865
Tognetti V, Joubert L (2011) On the influence of density functional approximations on some local Bader’s atoms-in-molecules properties. J Phys Chem A 115:5505–5515
Medvedev MG, Bushmarinov IS, Sun J, Perdew JP, Lyssenko KA (2017) Density functional theory is straying from the path toward the exact functional. Science 355:49–52
Schuster P (1969) LCAO-MO-Beschreibung intramolekularer Wasserstoffbrücken. Mh Chem 100:2084–2095
Schuster P, Schuster P, Zundel G, Sandorfy C (eds) (1976) The Hydrogen Bond, vol I. North-Holland, Amsterdam
Emsley J (1984) The Composition, Structure and Hydrogen Bonding of the β-Diketones. Struct Bond 57:147–191
Buemi G, Gandolfo C (1989) Malondialdehyde and Acetylacetone: An AM1 Study of their Molecular Structures and Keto-Enol Tautomerism. J Chem Soc. Faraday Trans 85:215– 227
Millefiori S, Di Bella S (1991) Hydrogen bonding and tautomerism in 3-substituted β-thioxoketones: an ab initio molecular orbital study. J Chem Soc. Faraday Trans 87:1297–1302
Luth K, Scheiner S (1994) Excited-State Energetics and Proton-Transfer Barriers in Malonaldehyde. J Phys Chem 98:3582–3587
Scheiner S, Kar T, C̆uma M (1997) Excited State Intramolecular Proton Transfer in Anionic Analogues of Malonaldehyde. J Phys Chem A 101:5901–5909
Cuma M, Scheiner S, Kar T (1999) Effect of adjoining aromatic ring upon excited state proton transfer, o-hydroxybenzaldehyde. J Mol Struct (Theochem) 467:37–49
Chung G, Kwon O, Kwon Y (1997) Theoretical Study on 1,2-Dihydroxybenzene and 2-Hydroxythiophenol: Intramolecular Hydrogen Bonding. J Phys Chem A 101:9415–9420
Rozas I, Alkorta I, Elguero J (2001) Intramolecular Hydrogen Bonds in ortho-Substituted Hydroxybenzenes and in 8-Susbtituted 1-Hydroxynaphthalenes: Can a Methyl Group Be an Acceptor of Hydrogen Bonds J Phys Chem A 105:10462–10467
Kovács A, Szabó A, Hargittai I (2002) Structural Characteristics of Intramolecular Hydrogen Bonding in Benzene Derivatives. Acc Chem Res 35:887–894
Grabowski SJ (2003) π-Electron delocalisation for intramolecular resonance assisted hydrogen bonds. J Phys Org Chem 16:797–802
Grabowski SJ (2004) Hydrogen bonding strength–measures based on geometric and topological parameters. J Phys Org Chem 17:18–31
Estácio SG, do Couto PC, Costa Cabral BJ, Minas da Piedade ME, Martinho Simões JA (2004) Energetics of Intramolecular Hydrogen Bonding in Di-substituted Benzenes by the ortho-para Method. J Phys Chem A 108:10833–10843
Groom CR, Bruno IJ, Lightfoot MP, Ward SC (2016) The Cambridge Structural Database. Acta Cryst B72:171–179
Popelier PLA, Logothetis G (1998) Characterization of an Agostic Bond on the Basis of the Electron Density. J Organomet Chem 555:101–111
Tognetti V, Joubert L, Raucoules R, De Bruin T, Adamo C (2012) Characterizing Agosticity Using the Quantum Theory of Atoms in Molecules: Bond Critical Points and Their Local Properties. J Phys Chem A 116:5472–5479
Fradera X, Austen MA, Bader RFW (1999) The Lewis Model and Beyond. J Phys Chem A 103:304–314
Wang YG, Matta C, Werstiuk NH (2003) Comparison of Localization and Delocalization Indices Obtained with Hartree-Fock and Conventional Correlated Methods: Effect of Coulomb Correlation. J Comput Chem 24:1720–1729
Acknowledgements
The National Science Centre of Poland is gratefully acknowledged for the financial support grant no. 2015/17/B/ST4/04050. We thank Dr. Marcin Palusiak from the University of Łódź for the search in the Cambridge Structural Database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jabłoński, M. Strength of Si–H ⋯ B charge-inverted hydrogen bonds in 1-silacyclopent-2-enes and 1-silacyclohex-2-enes. Struct Chem 28, 1697–1706 (2017). https://doi.org/10.1007/s11224-017-0939-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-0939-6