Abstract
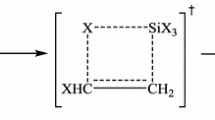
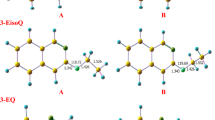
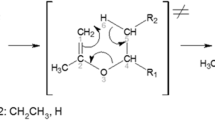
Decomposition of the three isomeric compounds, 3-buten-1-ol (1), 3-methoxy-1-propene (2), and ethoxyethene (3), at two different (300 and 550 K) temperatures has been investigated by means of ab initio molecular orbital theory (MP2/6-311+G**//B3LYP/6-311+G**), hybrid-density functional theory (B3LYP/6-311+G**), the complete basis set, nuclear magnetic resonance analysis, and the electrostatic model associated with the dipole–dipole interactions. All three levels of theory showed that the calculated Gibbs free energy differences between the transition and ground state structures (ΔG ≠) increase from compound 1 to compound 3. The variations of the calculated ΔG ≠ values can not be justified by the decrease of the calculated global hardness (η) differences between the ground and transition states structures (i.e., Δ[η(GS)−η(TS)]). Based on the synchronicity indices, the transition state structures of compounds 1–3 involve synchronous aromatic transition structures, but there is no significant difference between their calculated synchronicity indices. The optimized geometries for the transition state structures of the decomposition reactions of compounds 1–3 consist in chair-like six-membered rings. The variation of the calculated activation entropy (ΔS ≠) values can not be justified by the decrease of Δ[η(GS)−η(TS)] parameter from compound 1 to compound 3. On the other hand, dipole moment differences between the ground and transition state structures [Δ(µ TS−µ GS)] decrease from compound 1 to compound 3. Therefore, the electrostatic model associated with the dipole–dipole interactions justifies the increase of the calculated ΔG ≠ values from compound 1 to compound 3. The correlations between ΔG ≠, Δ[η(GS)−η(TS)], (ΔS ≠), k(T), electrostatic model, and structural parameters have been investigated.



Similar content being viewed by others
References
Arnold RT, Smolinsky G (1959) J Am Chem Soc 81:6443–6445
Arnold RT, Smolinsky G (1960) J Org Chem 25:129–130
Smith GG, Yates BL (1965) J Chem Soc 7242–7246
Henao D, Murillo J, Ruiz P, Quijano J, Mejía B, Castañeda L, Notario R (2012) J Phys Org Chem 25:883–887
Sakai Y, Ando H, Oguchi T, Murakami Y (2013) Chem Phys Lett 556:29–34
Quijano J, David J, Sanchez C, Rincon E, Guerra D, Leon LA, Notario R, Abboud JL (2002) J Mol Struct (Theochem) 580:201–205
Smith GG, Blau SE (1964) J Phys Chem 68:1231–1234
DePuy CH, King RW (1960) Chem Rev 60:431–457
Glasstone KJ, Laidler KJ, Erying H (1941) The theory of rate processes Chapter 4. McGraw-Hill, New York
Benson SW (1969) The foundations of chemical kinetics. McGraw-Hill, New York
Alder K, Pascher F, Schmitz A (1943) Chem Ber 76:27–53
Hoffmann HMR (1969) Angew Chem Int Ed Engl 8:556–557
Lopez V, Quijano J, Luna S, Ruiz P, Rios D, Parra W, Zapata E, Gaviria J, Notario R (2013) Struct Chem 24:1811–1816
Blades AT, Murphy GW (1952) J Am Chem Soc 74:1039–1041
Shimofuji K, Saito K, Imamura A (1991) J Phys Chem 95:155–165
Ibuki T, Takezaki Y (1977) Int J Chem Kinet 9:201–213
Chamorro E, Quijano J, Notario R, Sánchez C, León LA, Chuchani G (2003) Int J Quant Chem 91:618–625
Hehri WJ, Radom L, PvR Scheleyer, Pople JA (1986) Ab initio molecular orbital theory. Wiley, New York
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA (1999) J Chem Phys 110:2822–2827
Seminario JM, Politzer P (eds) (1995) Modern density function theory, a tool for chemistry. Elsevier, Amsterdam
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
McIver JW Jr., (1974) Acc Chem Res 7:72–84
Ermer O (1975) Tetrahedron 31:1849–1854
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) J Comput Chem 17:49–56
Fukui K (1970) J Phys Chem 74:4161–4163
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann Jr., RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Head-Gordon M, Replogle ES, Pople JA (1998) GAUSSIAN 98, Revision A.3, Gaussian Inc., Pittsburgh, PA
Schleyer PVR, Maerker C, Dransfeld A, Jiao H, Hommes NJVE (1996) J Am Chem Soc 118:6317–6318
Tapu D, Dixon DA, Roe C (2009) Chem Rev 109:3385–3407
Glasstone KJ, Laidler KJ, Eyring H (1941) The theory of rate processes, Chap 4. McGraw-Hill, New York
Pearson RG, Palke WE (1992) J Phys Chem 96:3283–3285
Chattaraj PK, Gutiérrez-Oliva S, Jaque P, Toro-Labbé A (2003) Mol Phys 101:2841–2853
Ghanty TK, Ghosh SK (2002) J Phys Chem A 106:4200–4204
Perez P, Toro-Labbe A (2000) J Phys Chem A 104:1557–1562
Borden WT, Loncharich RJ, Houk KN (1988) Annu Rev Phys Chem 39:213–236
Dewar MJS (1959) Tetrahedron Lett 16–18
Benchouk W, Mekelleche SM (2008) J Mol Struct (THEOCHEM) 862:1–6
Moyano A, Pericas MA, Valenti EA (1989) J Org Chem 54:573–582
Lecea B, Arrieta A, Roa G (1994) J Am Chem Soc 116:9613–9619
Morao I, Lecea B, Cossio FP (1997) J Org Chem 62:7033–7036
Cossio FP, Morao I, Jiao H, Schleyer PVR (1999) J Am Chem Soc 121:6737–6746
Acknowledgment
This work has been supported by the research grant from the Research Council of the Ahvaz Branch, Islamic Azad University. We thank Dr. Daryoush Tahmasebi for CBS-4 calculations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hasanzadeh, N., Nori-Shargh, D., Kayi, H. et al. Correlations between hardness, electrostatic interactions, and thermodynamic parameters in the decomposition reactions of 3-buten-1-ol, 3-methoxy-1-propene, and ethoxyethene. Struct Chem 26, 547–554 (2015). https://doi.org/10.1007/s11224-014-0514-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0514-3