Abstract
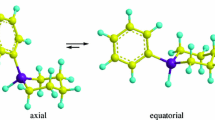
The conformational preference of the t-butyl group in 1-t-Bu-1-silacyclohexane was studied experimentally by means of gas-phase electron diffraction (GED), and temperature-dependent Raman spectroscopy as well as by quantum chemical calculations applying density functional theory and ab initio methods. According to the GED experiment at 283 K, the vapor of the title compound contains only the equatorial conformer. At 99.5 % level of confidence, up to 4 % of axial conformer cannot be completely excluded, however. The Raman spectroscopy experiment in the temperature range of 295–375 K of the neat liquid indicated that the equatorial conformer is favored over the axial one by 0.56 (15) kcal mol−1 (ΔH values). The experimental values are fairly well reproduced by the calculations. CCSD(T) calculations predict the equatorial conformer to have a 1.19 kcal mol−1 lower Gibbs free energy (corresponding to about 89 % equatorial preference) and a 0.99 kcal mol−1 lower enthalpy than the axial conformer at 300 K. According to natural bond orbital analysis, the equatorial conformer of the title compound is an example of a molecular stabilization, which is not favored by steric and conjugation effects but favored by electrostatic interactions. Results from dynamic NMR experiments were inconclusive.






Similar content being viewed by others
References
Eliel EL, Wilen SH (1994) Stereochemistry of organic compounds. Wiley, New York
Juaristi E (ed) (1995) Conformational behavior of six-membered rings. Methods in stereochemical analysis. VCH, New York
Arnason I, Kvaran Á, Bodi A (2006) Int J Quantum Chem 106:1975–1978
Winstein S, Holness NJ (1955) J Am Chem Soc 77:5562
Burkert U, Allinger NL (1982) Molecular mechanics, vol 177. ACS Monographs. American Chemical Society, Washington, DC
Booth H, Everett JR (1980) J Chem Soc Perkin Trans II:255–259
Bushweller CH (1995) Stereodynamics of cyclohexane and substituted cyclohexanes. Substituent A values. In: Juaristi E (ed) Conformational behavior of six-membered rings. Methods in stereochemical analysis. VCH, New York, pp 25–58
Manharan M, Eliel EL (1984) Tetrahedron Lett 25(31):3267–3268
Wiberg KB, Hammer JD, Castejon H, Bailey WF, DeLeon EL, Jarret RM (1999) J Org Chem 64:2085–2095
Taddei F, Kleinpeter E (2004) J Mol Struct (THEOCHEM) 683:29–41
Taddei F, Kleinpeter E (2005) J Mol Struct (Theochem) 718:141–151
Cortés-Guzmán F, Hernández-Trujillo J, Cuevas G (2003) J Phys Chem A 107(44):9253–9256
Arnason I, Kvaran A, Jonsdottir S, Gudnason PI, Oberhammer H (2002) J Org Chem 67(11):3827–3831
Favero LB, Velino B, Caminati W, Arnason I, Kvaran A (2006) Organometallics 25:3813–3816
Kern T, Hölbling M, Dzambaski A, Flock M, Hassler K, Wallevik SÓ, Arnason I, Bjornsson R (2012) J Raman Spectrosc 43(9):1337–1342
Shainyan BA, Kleinpeter E (2012) Tetrahedron 68:114–125
Durig JR, El Defrawy AM, Ward RM, Guirgis GA, Gounev TK (2008) Struct Chem 19:579–594
Eliel EL, Manoharan M (1981) J Org Chem 46:1959–1962
Squillacote ME, Neth JM (1987) J Am Chem Soc 109:198–202
Girichev GV, Giricheva NI, Bodi A, Gudnason PI, Jonsdottir S, Kvaran A, Arnason I, Oberhammer H (2007) Chem Eur J 13:1776–1783
Girichev GV, Giricheva NI, Bodi A, Gudnason PI, Jonsdottir S, Kvaran A, Arnason I, Oberhammer H (2009) Chem Eur J 15:8929. doi:10.1002/chem.200902290
Wallevik SÓ, Bjornsson R, Kvaran Á, Jonsdottir S, Arnason I, Belyakov AV, Baskakov AA, Hassler K, Oberhammer H (2010) J Phys Chem A 114(5):2127–2135
Wallevik SÓ, Bjornsson R, Kvaran Á, Jonsdottir S, Arnason I, Belyakov AV, Kern T, Hassler K (2013) Organometallics 32:6996–7005
Shainyan BA, Kleinpeter E (2013) Tetrahedron 69:5927–5936
Bjornsson R, Arnason I (2009) Phys Chem Chem Phys 11(39):8689–8697. doi:10.1039/b9100116d
Sigolaev YF, Semenov SG, Belyakov AV (2013) Russ J Gen Chem 83:932–937. doi:10.1134/S1070363213050083
Sipachev VA (1985) J Mol Struct (Theochem) 121:143–151
Sipachev VA (1999) Vibrational effects in diffraction and microwave experiments: a start on the problem, vol 5. JAI, New York
Hamilton WC (1965) Acta Crystallogr 18:502–510
Bodi A, Kvaran Á, Jonsdottir S, Antonsson E, Wallevik SÓ, Arnason I, Belyakov AV, Baskakov AA, Hölbling M, Oberhammer H (2007) Organometallics 26(26):6544–6550
Badenhoop JK, Weinhold F (1997) J Chem Phys 107(14):5406
Badenhoop JK, Weinhold F (1997) J Chem Phys 107(14):5422
Badenhoop JK, Weinhold F (1999) Int J Quantum Chem 72(4):269
Weinhold F (2012) Discovering chemistry with natural bond orbitals. Wiley, Hoboken
Kong L, Bischoff FA, Valeev EF (2012) Chem Rev 112:75–107
West R (1954) J Am Chem Soc 76:6012–6014
Girichev GV, Shlykov SA, Petrova VN, Subbotina NY, Lapshina SB, Danilova TG (1988) Izv Vyssh Uchebn Zaved, Khim Khim Tekhnol (in Russian) 31:46
Girichev GV, Shlykov SA, Revichev YF (1986) Instrum Exp Tech (English Transl) 2 (1984) 457(4):167
Girichev GV, Utkin AN, Revichev YF (1984) Instrum Exp Tech (English Transl) 2 (1984) 457(N2):187
Girichev EG, Zakharov AV, Girichev GV, Bazanov MI (2000) Izv VUZ Tekstiln Prom (Russian) 2:142
Ross AW, Fink M, Hilderbrandt RL (1992) International Tables of Crystallography, C. Kluwer, Dordrecht
Andersen B, Seip HM, Strand TG, Stolevik R (1969) Acta Chem Scand 23:3224
Gundersen G, Samdal S, Seip HM (1981) Least squares structural refinement program based on gas electron-diffraction data, vol I–III. Department of Chemistry, University of Oslo, Oslo
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts RE, Stratmann O, Yazyev AJ, Austin R, Cammi C, Pomelli JW, Ochterski R, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox D (2009) Gaussian 09, Revision D01. Gaussian, Inc., Wallingford CT
Glendening ED, Landis CR, Weinhold F (2012) WIREs Comput Mol Sci 2:1–42
Weinhold F (1998) Natural bond orbital methods. Encyclopedia of computational chemistry, vol 3. Wiley, Chichester
Weinhold F, Landis CR (2005) Valency and bonding: a natural bond orbital donor-acceptor perspective. Cambridge University Press, Cambridge
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Landis CR, Weinhold F (2013) Natural bond orbital analysis program: NBO 6.0. Theoretical Chemistry Institute, University of Wisconsin, Madison, WI
Glendening ED, Landis CR, Weinhold F (2013) J Comput Chem 34:1429–1437. doi:10.1002/jcc.23266
Peterson KA, Adler TB, Werner HJ (2008) J Chem Phys 128:084102
Yousaf KE, Peterson KA (2008) J Chem Phys 129:184108
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7(18):3297–3305
Acknowledgments
A financial support from RANNÍS—The Icelandic Centre for Research (Grant No. 080038021) is gratefully acknowledged. A.V.B. is grateful to the Government of RF (Project 11.G34.31.0069). S.A.S. is grateful to the Russian Foundation for Basic Research, RFBR (Grant No. 14-03-00923-a). T.K. and K.H. thank the Austrian Science Foundation FWF (Fonds zur Förderung der wissenschaftlichen Forschung, Vienna) for financial support of project P 21272-N19.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
11224_2014_503_MOESM1_ESM.docx
Van´t Hoff plots using relative band heights and band areas, respectively. MP2-cc-pVTZ optimized geometries. Supplementary material 1 (DOCX 134 kb)
Rights and permissions
About this article
Cite this article
Belyakov, A.V., Sigolaev, Y., Shlykov, S.A. et al. Conformational properties of 1-tert-butyl-1-silacyclohexane, C5H10SiH(t-Bu): gas-phase electron diffraction, temperature-dependent Raman spectroscopy, and quantum chemical calculations. Struct Chem 26, 445–453 (2015). https://doi.org/10.1007/s11224-014-0503-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0503-6