Abstract
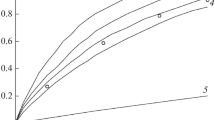
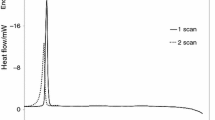
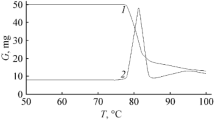
Thermal decomposition of C,N-disubstituted gem-dinitroethylnitramines in dilute solutions in inert solvents is a first-order reaction and it is unaffected by the polarity of the solvent. The rate constant is largely controlled by the steric effect of the substituent at the carbon atom bearing gem-nitro groups. A correlation is found between the rate constant and the steric constant E s of the substituent. This correlation permits prediction not only of thermal stability of yet unexplored compounds, but also of change of the decomposition mechanism.
Similar content being viewed by others
REFERENCES
Manelis, G.B., Nazin, G.M., Rubtsov, Yu.I., and Strunin, V.A., Termicheskoe razlozhenie i gorenie vzryvchatykh veshchestv i porokhov (Thermal Decomposition and Combustion of Explosives and Powders), Moscow: Nauka, 1996.
Nazin, G.M. and Manelis, G.B., Usp, Khim., 1994, vol. 63, no.4, p. 327.
Flournoy, J.M., J. Chem. Phys., 1962, vol. 36, no.4, p. 1107.
Polyakova, A.A. and Khmelnitskii, R.A., Mass-spektrometriya v organicheskoi khimii (Mass Spectrometry in Organic Chemistry), Leningrad: Khimiya, 1972.
Khrapkovskii, G.M., Shamov, A.G., Shamov, G.A., and Shlyapochnikov, V.A., Ross. Khim. Zh., 1997, vol. 41, no.4, p. 14.
Palm, V.A., Osnovy kolichestvennoi teorii organicheskikh reaktsii (Fundamentals of Quantitative Theory of Organic Reactions), Leningrad: Khimiya, 1977.
Shan’ko, V.N., Stepanov, R.S., and Gidaspov, B.V., Materialy konferentsii po itogam nauchno-issledovatel’skikh rabot. Sektsiya organicheskoi khimii (Proc. Conf. on the Results of Research Works. Section of Organic Chemistry), Krasnoyarsk: Stavropol. Tekhnol. Inst., 1971, p. 41.
Stepanov, R.S., Shan’ko, V.N., Medvetskaya, I.P., and Gorodetskaya, V.M., Khimicheskaya fizika protsessov goreniya i vzryva. Kinetika khimicheskikh reaktsii (Chemical Physics of the Processes of Combustion and Explosion. Kinetics of Chemical Reactions), Chernogolovka: Inst. Obshch. Neorg. Fiz. Akad. Nauk SSSR, 1977, p. 56.
Korsunskii, B.L., Matveev, V.G., Nazina, L.D., and Nazin, G.M., Izv. Ross. Akad. Nauk, Ser. Khim., 1998, no. 2, p. 259.
Frankel, M.B. and Klager, K., J. Chem. Eng. Data, 1962, no 7, p. 412.
Feuer, H. and Swarts, W.A., J. Org. Chem., 1962, vol. 27, p. 1455←1459.
Stepanov R.S., Fiziko-khimicheskie ispytaniya vzryvchatykh veshchestv (Physicochemical Tests of Explosives), Krasnoyarsk: Krasnoyarsk. Politekh. Inst., 1989.
Author information
Authors and Affiliations
Additional information
Translated from Zhurnal Obshchei Khimii, Vol. 74, No. 10, 2004, pp. 1669–1673.
Original Russian Text Copyright © 2004 by Stepanov, Kruglyakova, Astakhov.
Rights and permissions
About this article
Cite this article
Stepanov, R.S., Kruglyakova, L.A. & Astakhov, A.M. Thermal decomposition of gem-dinitroethylnitramines. Russ J Gen Chem 74, 1547–1551 (2004). https://doi.org/10.1007/s11176-005-0053-0
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11176-005-0053-0