Abstract
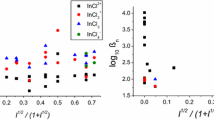
Complex formation in Ln chloride solutions is studied by spectrophotometric method. Electronic absorption spectra of Nd3+, Sm3+, and Ho3+ ions are measured in the range of supersensitive transitions in solution with Cl− ion concentration from 0 to 5 mol/l in 100–250°C temperature interval under saturated vapor pressure. The Nd and Sm spectra represent integrated curves that mainly consist of Ln3+ and LnCl2+ absorption bands (with stability constant β1), while the Ho spectra consist of Ho3+ and HoCl +2 absorption bands (with β2). The stability constants β1 and β2 calculated for each wave number by linear regression method acquire steady values and have the meaning of the best unbiased linear estimates. Thermodynamic values of logβ1 for Nd, Sm, and Ho monochlorides lie in a narrow interval at constant temperature. In the case of Nd and Sm, the temperature curves of logβ1 and logβ2 have smaller slopes as compared to that of Ho, which is explained by the effect of a covalent component in their spectra that adds to the ionic nature of the bonds in monochloride complexes. The β2 values increase in the order Nd<Sm<Ho in accordance with electrostatic model of a bond.
Similar content being viewed by others

REFERENCES
Mal’kova, T.V., Shutova, G.A., and Yatsimirskii, K.B., Zh. Neorg. Khim., 1964, vol. 9, no.8, p. 1833.
Kozachenko, N.N., Batyaev, I.M., and Mironov, V.E., Zh. Neorg. Khim., 1970, vol. 15, no.3, p. 888.
Mironov, V.E. and Avramenko, V.I., Koord. Khim., 1982, vol. 8, no.8, p. 636.
Romanenko, E.O. and Kostromina, N.A., Zh. Neorg. Khim., 1967, vol. 12, no.8, p. 516.
Davidenko, N.K., Luzhina, L.N., and Yatsimirskii, K.B., Zh. Neorg. Khim., 1972, vol. 178, no.1, p. 636.
Mal’kova, T.V., Shutova, G.A., and Yatsimirskii, K.B., Zh. Neorg. Khim., 1966, vol. 11, no.7, p. 1556.
Selwood, P.W., J. Am. Chem. Soc., 1930, vol. 52, p. 4308.
Judd, B.R., Lanthanide and Actinide Chemistry and Spectroscopy, Washington, DC: Am. Chem. Soc, 1980, p. 267.
Poluektov, N.S., Kononenko, L.I., Efryushina, N.P., and Bel’tyukova, S.V., Spektrofotometricheskie i lyuminestsentnye metody opredeleniya lantanoidov (Spectrophotometric and Luminescence Methods for Determination of Lanthanides), Kiev: Naukova Dumka, 1989.
Bell, J.T., Thompson, C.C., and Helton, D.M., J. Chem. Soc., 1969, vol. 73, no.19, p. 3338.
Wood, S.A., Chem. Geol., 1990, vol. 73, no.19, p. 3338.
Haas, J.R., Shock, E.L., and Sassani, D.C., Geochim. Cosmochim. Acta, 1995, vol. 59, no.21, p. 4325.
Gammons, C.H., Wood, S.A., and Williams-Jones, A.E., Geochim. Cosmochim. Acta, 1996, vol. 60, no.23, p. 4615.
Bakhshiev, N.G., Spektroskopiya mezhmolekulyarnykh vzaimodeistvii (Spectroscopy of Intermolecular Interactions), Leningrad: Nauka, 1972.
Stepanchikova, S.A., Dokl. Akad. Nauk, 1999, vol. 369, p. 517.
Bersuker, I.B., Elektronnoe stroenie i svoistva koordinatsionykh soedinenii (Electronic Structure and Properties of Coordination Compounds), Leningrad: Khimiya, 1976.
Sviridov, D.E. and Smirnov, Yu.F., Teoriya opticheskikh spektrov ionov perekhodnykh metallov (Theory of Optical Spectra of Transition Metal Ions), Moscow: Nauka, 1977.
Judd, B.R., Phys. Rev., 1962, vol. 127, no.3, p. 750.
Offelt, G.S., J. Chem. Phys., 1962, vol. 37, no.3, p. 511.
Bel’tyukova, S.V., Poluektov, N.S., and Nazarenko, N.A., Dokl. Akad. Nauk SSSR, 1982, vol. 264, no.5, p. 1146.
Poluektov, N.S., Alakaeva, L.A., and Tishchenko, M.A., Zh. Prikl. Spectrosk., 1972, vol. 17, no.5, p. 819.
Yatsimirskii, K.B., Kostromina, N.A., Sheka, Z.A., et al., Khimiya kompleksnykh soedinenii redkozemel’nykh elementov (Chemistry of Rare-Earth Complex Compounds), Kiev: Naukova Dumka, 1966.
Helgeson, H.C., Am. J. Sci., 1966, vol. 267, no.7, p. 729.
Naumov, G.B., Ryzhenko, B.N., and Khodakovskii, I.L., Spravochnik termodinamicheskikh velichin (Thermodynamic Data Handbook), Moscow: Atomizdat, 1971.
Bryzgalin, O.V., Geokhimiya, 1985, no. 8, p. 1184.
Tagirov, B.R., Zotov, A.V., and Akinfiev, N.N., Geochim. Cosmochim. Acta, 1967, vol. 61, no.29, p. 4267.
El’yashevich, M.A., Atomnaya i molekulyarnaya spektroskopiya (Atomic and Molecular Spectroscopy), Moscow: Gos. Izd. Fiz.-mat. Lit, 1962.
Belevantsev, V.I. and Malkova V.I., Pryamye i obratnye zadachi khimicheskoi termodinamiki (Direct and Inverse Problems of Chemical Thermodynamics), Novosibirsk: Nauka, 1987.
Author information
Authors and Affiliations
Additional information
Translated from Koordinatsionnaya Khimiya, Vol. 31, No. 3, 2005, pp. 207–217.
Original Russian Text Copyright © 2005 by Stepanchikova, Kolonin.
Rights and permissions
About this article
Cite this article
Stepanchikova, S.A., Kolonin, G.R. Spectrophotometric study of Nd, Sm, and Ho complexation in chloride solutions at 100–250°C. Russ J Coord Chem 31, 193–202 (2005). https://doi.org/10.1007/s11173-005-0076-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11173-005-0076-4