Abstract
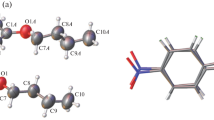
4-(Tetradecyloxy)phenacyl thiocyanate NCS—CH2—C(O)—C6H4—OC14H29 was studied by differential scanning calorimetry (DSC) and X-ray diffraction. According to the DSC analysis, this compound does not form a mesophase upon melting or cooling of the isotropic melt. The crystal-I—crystal-II phase transition with a small enthalpy, which is not accompanied by mechanical degradation of the crystal, occurs at 68.8 °C prior to the melting of the crystals (82.5 °C). The crystal and molecular structure of the compound was studied by X-ray diffraction at temperatures below (22 °C) and above (73 °C) the temperature of the phase transition in the crystal. Both crystalline phases have nearly identical structures. The crystal packing consists of alternating loosely packed aliphatic and closely packed aromatic regions, which is typical of crystals of mesomorphic compounds. In the aromatic regions of the crystal packing, there are intermolecular S…S contacts. In the low-temperature phase crystal-I, the S…S distances equal to 3.458 Å correspond to a non-covalent interaction, through which the molecules are linked into centrosymmetric dimers. In the high-temperature phase, the S…S distances are increased to 3.623 Å, which corresponds to twice the van der Waals radius of sulfur. The absence of mesomorphism in the compound is attributed to the fact that, upon heating, the crystal undergoes a transition to another phase, which contains no structural elements responsible for the structuring of the melt.
Similar content being viewed by others
References
L. G. Kuz’mina, P. Kalle, A. V. Churakov, Russ. Chem. Bull., 2020, 69, 1054.
I. I. Konstantinov, A. V. Churakov, L. G. Kuz’mina, Crystallogr. Reports, 2013, 58, 81.
L. G. Kuz’mina, M. A. Gunina, A. V. Churakov, S. M. Pestov, Crystallogr. Reports, 2013, 58, 253.
L. G. Kuz’mina, I. I. Konstantinov, E. K. Lermontova, Mol. Cryst. Liq. Cryst., 2014, 588, 1.
L. G. Kuz’mina, I. I. Konstantinov, S. I. Bezzubov, High Energy Chem., 2016, 50, 453.
L. G. Kuz’mina, I. I. Konstantinov, A. V. Churakov, M. A. Navasardyan, Acta Cryst. E, 2017, 73, 1052.
L. G. Kuz’mina, I. I. Konstantinov, A. V. Churakov, Mol. Cryst. Liq. Cryst., 2018, 664, 95.
L. G. Kuz’mina, M. A. Navasardyan, I. I. Konstantinov, Crystallogr. Reports, 2019, 64, 76.
B. Donnio, S. Buathong, I. Bury, D. Guillon, Chem. Soc. Rev., 2007, 36, 1495.
O. Lehman, Uber flussige Kristalle, Z. Phys. Chem., 1889, 4, 462.
W. Maier, A. Saupe, Z. Naturforsch, 1960, 15a, 287.
R. L. Humphrie, P. G. Jame, G. R. Luckhurs, J. Chem. Soc., Faraday Trans. 2, 1972, 68, 1031.
P. G. De Gennes, Mol. Cryst. Liq. Cryst., 1973, 21, 49.
W. L. Mc Millan, Phys. Rev. A, 1973, 8, 1921.
A. Wulf, Phys. Rev. A, 1975, 11, 365.
M. A. Cotter, Mol. Cryst. Liq. Cryst., 1983, 97, 29.
M. A. Osipov, Molecular Theories of Liquid Crystals. Sections 2, Ch. III, V. 1, in Handbook of Liquid Crystals, Eds D. Demus, J. Goodby, G. W. Gray, H.-W. Spies, V. Vill, Weinheim, Wiley-VCH, 1998, p. 40.
G. Vertoge, W. H. Jeu, Thermotropic Liquid Crystals, Fundamentals, Springer-Verlag, Berlin, 1988, 324.
P. G. de Gennes, J. Prost, The Physics of Liquid Crystals, Oxford University Press, New York, 1995, 616 p.
S. Singh, Phys. Rep., 2000, 324, 2–4, 107.
C. R. Groon, F. H. Allen, Angew. Chem., 2014, 53, 662.
SAINT. Version 6.02A, Bruker AXS, Madison, W1, 2001.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Pushman, J. Appl. Crystallogr., 2009, 42, 339.
Author information
Authors and Affiliations
Corresponding author
Additional information
The X-ray diffraction study was performed using equipment of the Shared Facility Center of the N. S. Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences.
This work was financially supported by the Russian Science Foundation (Project No. 16-13-10273).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 32–38, January, 2021.
Rights and permissions
About this article
Cite this article
Kuz’mina, L.G., Churakov, A.V., Navasardyan, M.A. et al. Crystal packing features of potentially mesomorphic organic compounds; phase transitions in 4-(tetradecyloxy)phenacyl thiocyanate NCS-CH2-C(O)-C6H4-OC14H29. Russ Chem Bull 70, 32–38 (2021). https://doi.org/10.1007/s11172-021-3053-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3053-2