Abstract
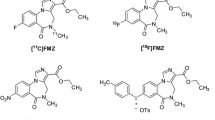
An express method of solid-phase extraction was proposed for the first time for isolation and purification of [18F]flumazenil, a radioligand used to quantify the density of central benzodiazepine receptors by positron emission tomography (PET). This novel approach afforded the radioligand with >97% radiochemical purity and a high chemical purity (nitromazenil content <1 μg mL–1) and considerably reduced the time of the synthesis (from 90 to 50 min). The nonoptimized decay-corrected radiochemical yield was 8%, and the specific radioactivity was >37 GBq μmol–1. The novel synthetic procedure easily can be integrated into automatic modules for the synthesis of clinically used PET radiopharmaceuticals.
Similar content being viewed by others
References
W. D. Heiss, K. Herholz, J. Nucl. Med., 2006, 47, 302.
S. M. Ametamey, P. A. Schubiger, Nucl. Sci. Tech., 2006, 17, 143.
J. D. Andersson, C. Halldin, J. Labelled Compd. Radiopharm., 2013, 56, 196.
P. H. Elsinga, Nucl. Med. Rev., 2012, 15 (Suppl. C), 13.
J. Y. Njiwa, K. R. Gray, N. Costes, F. Mauguiere, P. Ryvlin, A. Hammers, NeuroImage: Clin., 2015, 7, 122.
T. Geeraerts, J. P. Coles, F. I. Aigbirhio, J. D. Pickard, D. K. Menon, T. D. Fryer, Y. T. Hong, Ann. Nucl. Med., 2011, 25, 396.
S. M. Moerlein, J. S. Perlmutter, Eur. J. Pharmacol., 1992, 218, 109.
P. Leveque, S. Sanabria-Bohorquez, A. Bol, A. De Volder, D. Labar, K. Van Rijckevorse, B. Gallez, Eur. J. Nucl. Med. Mol. Imaging, 2003, 30, 1630.
Y. H. Yoon, J. M. Jeong, W. H. Kim, S. H. Hong, Y. Lee, H. S. Kil, M. C. Lee, Nucl. Med. Biol., 2003, 30, 521.
M. Mitterhauser, W. Wadsak, L. Wabnegger, L. K. Mien, S. Tögel, O. Langer, W. Sieghart, H. Viernstein, K. Kletter, R. Dudczak, Nucl. Med. Biol., 2004, 31, 291.
Y. S. Chang, J. M. Jeong, Y. H. Yoon, W. J. Kang, S. J. Lee, D. S. Lee, J.-K. Chung, M. C. Lee, Nucl. Med. Biol., 2005, 32, 263.
H.-J. Park, C. H. Kim, E. S. Park, B. Park, S. R. Oh, M.-K. Oh, Ch. Park, J. D. Lee, J. Nucl. Med., 2013, 54, 1263.
N. N. Ryzhikov, N. A. Gomzina, O. S. Fedorova, D. A. Vasilév, A. P. Kostikov, R. N. Krasikova, Radiochemistry, 2004, 46, 290.
J. Delforge, L. Spelle, B. Bendriem, Y. Samson, M. Bottlaender, S. Papageorgiou, A. Syrota, J. Nucl. Med., 1996, 37, 5.
N. N. Ryzhikov, N. Seneca, R. N. Krasikova, N. A. Gomzina, E. Shchukin, O. S. Fedorova, D. A. Vassiliev, B. Gulyás, H. Hall, I. Savic, C. Halldin, Nucl. Med. Biol., 2005, 32, 109.
G. Massaweh, E. Schirrmacher, C. La Fougere, M. Kovacevic, C. Wängler, D. Jolly, P. Gravel, A. J. Reader, A. Thiel, R. Schirrmacher, Nucl. Med. Biol., 2009, 36, 721.
B. S. Moon, H. S. Kil, J. H. Park, J. S. Kim, J. Park, D. Y. Chi, B. C. Lee, S. E. Kim, Org. Biomol. Chem., 2011, 9, 8346.
R. Wong, R. Iwata, H. Saiki, S. Furumoto, Y. Ishikawa, E. Ozeki, Appl. Radiat. Isot., 2012, 70, 193.
K. S. Mandap, T. Ido, Y. Kiyono, M. Kobayashi, T. G. Lohith, T. Mori, S. Kasamatsu, T. Kudo, H. Okazawa, Y. Fujibayashi, Nucl. Med. Biol., 2009, 36, 403.
I. Odano, C. Halldin, P. Karlsson, A. Varrone, A. J. Airaksinen, R. N. Krasikova, L. Farde, Neuroimage, 2009, 45, 891.
S. Dedeurwaerdere, M.-K. Gregoire, L. Vivash, P. Roselt, D. Binns, C. Fookes, I. Greguric, T. Pham, C. Loc´h, A. Katsifis, R. J. Hicks, T. J. O´Brien, D. E. Myers, Eur. J. Nucl. Med. Mol. Imaging, 2009, 36, 958.
L. Vivash, M.-K. Gregoire, V. Bouilleret, A. Berard, C. Wimberley, D. Binns, P. Roselt, A. Katsifis, D. E. Myers, R. J. Hicks, T. J. O´Brien, S. Dedeurwaerdere, PLoS One, 2014, 9, 86722.
R. Schirrmacher, A. Kostikov, H. Massaweh, M. Kovacevic, K. Wangler, A. Thiel, in Radiopharmaceuticals for Positron Emission Tomography, Eds P. J. H. Scott, B. G. Hockley, John Wiley and Sons, New York, 2012, p. 111.
B. S. Moon, J. H. Park, H. J. Lee, B. C. Lee, S. E. Kim, Mol. Imaging Biol., 2014, 16, 619.
A. Jackson, M. R. Battle, D. M. O´Shea, W.-F. Chau, A. Gaeta, S. L. Brown, A. L. Ewan, C. L. Jones, P. A. Jones, J. L. Woodcraft, D. R. Bouvet, B. B. Guilbert, W. Trigg, Nucl. Med. Biol., 2014, 41, 196.
D. Ory, J. Van den Brane, T. de Groot, K. Serdons, M. Bex, L. Declercq, F. Cleeren, M. Ooms, K. Van Laere, A. Verbruggen, G. Bormans, J. Pharm. Biomed. Anal., 2015, 111, 209.
Gosudarstvennaya farmakopeya RF [State Pharmacopoeia of the Russian Federation], XII ed., Scientific Center for Drug Appraisal, Moscow, 2008, 704 pp. (in Russian)
European Pharmacopoeia 8.0, Strasbourg, France, 2008, p. 1054.
S. K. Chitneni, K. Serdons, N. Evens, H. Fonge, S. Celen, Ch. M. Deroose, Z. Debyser, L. Mortelmans, A. M. Verbruggen, G. M. Bormans, J. Chromatogr. A, 2008, 1189, 323.
C. R. Mallet, J. R. Mazzeo, U. Neue, Rapid Commun. Mass Spectrom., 2001, 15, 1075.
S. J. Lee, J. S. Hyun, S. J. Oh, K. H. Yu, J. S. Ryu, J. Labelled Compd. Radiopharm., 2013, 56, 731.
L. Xiong, R. Wang, C. Liang, X. Teng, F. Jiang, L. Zeng, H. Ye, C. Ni, X. Yuan, Y. Rao, Y. Zhang, J. Chromatogr. A, 2015, 1395, 99.
B. H. Mock, W. Winkle, M. T. Vawrek, Nucl. Med. Biol., 1997, 24, 193.
Guidance for Industry Genotoxic and Carcinogenic Impurities in Drug Substances and Products: Recommended Approaches, U.S. Department of Health and Human Services FDA for CDER, 2008, 16 pp.
H. Sun, S. G. Di Magno, J. Fluorine Chem., 2007, 128, 806.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences N. S. Zefirov on the occasion of his 80th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 0794—0800, March, 2016.
Rights and permissions
About this article
Cite this article
Nasirzadeh, M., Vaulina, D.D., Kuznetsova, O.F. et al. A novel approach to the synthesis of [18F]flumazenil, a radioligand for PET imaging of central benzodiazepine receptors. Russ Chem Bull 65, 794–800 (2016). https://doi.org/10.1007/s11172-016-1376-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-016-1376-1