Abstract
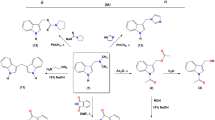
Toxicity, antioxidant activity, and membrane protective properties were studied on mammalian red blood cells for carboxy derivatives and esters of 2,6-diisobornyl-4-methylphenol in comparison with both diastereomers (meso-form and racemate) of this terpenophenol. The studies showed that all the synthesized compounds posses an antioxidant activity, however, the cytotoxicity of derivatives with a free carboxy group might be an obstacle to the use of these compounds in pharmacology. The most promising compound is methyl 4-hydroxy-3,5-diisobornylbenzoate distinguished by a low toxicity and high antioxidant and membrane protective activity, which is comparable to that of 2,6-diisobornyl-4-methylphenol.
Similar content being viewed by others
References
O. I. Kiselev, Khimiopreparaty i khimioterapiya grippa [Chemotherapy Agents and Chemotherapy of Influenza], Rostok, St.-Petersburg, 2012, 272 pp. (in Russian).
M. Cirri, P. Mura, P. Corvi Mora, Int. J. Pharm., 2007, 340, 84.
M. D. Mashkovskii, Lekarstvennye sredstva [Medicines], 14th ed., Novaya Volna, Moscow, 2000, V. 1, 540 pp. (in Russian).
A. T. Soldatenkov, N. M. Kolyadina, I. V. Shendrik, Osnovy organicheskoi khimii lekarstvennykh veshchestv [Principles of Organic Chemistry of Medicinal Agents], Mir, BINOM. Laboratoriya Znanii, Moscow, 2007, 191 pp. (in Russian).
M. B. Plotnikov, V. I. Smol´yakova, I. S. Ivanov, A. V. Kuchin, I. J. Chukicheva, E. V. Buravlev, E. A. Krasnov, Pharm. Chem. J. (Engl. Transl.), 2011, 44, 530 [Khim. Farm. Zh., 2010, 44, No. 10, 9].
M. B. Plotnikov, V. I. Smolyakova, I. S. Ivanov, G. A. Chernisheva, A. V. Kuchin, I. J. Chukicheva, E. A. Krasnov, Bull. Exp. Biol. Med. (Engl. Transl.), 2010, 149, 721 [Byul. Eksperim. Biol. Med., 2010, 149, 660].
S. V. Logvinov, M. B. Plotnikov, A. A. Zhdankina, V. I. Smolyakova, I. S. Ivanov, A. V. Kuchin, I. Yu. Chukicheva, E. Yu. Varakuta, Neurosci. Behav. Physiol. (Engl. Transl.), 2010, 40, 779 [Morfologiya, 2009, 136, No. 5, 42].
E. V. Buravlev, I. Yu. Chukicheva, K. Yu. Suponitskii, A. V. Kutchin, Russ. J. Org. Chem. (Engl. Transl.), 2013, 49, 60 [Zh. Org. Khim., 2013, 49, 69].
N. V. Pizova, Nevrologiya, neiropsikhiatriya, psikhosomatika [Neurology, Neuropsychiatry, Psychosomatics], 2010, No. 1, 67 (in Rusian).
M. A. Torlopov, E. V. Buravlev, O. V. Sukrusheva, I. Yu. Chukicheva, Russ. Chem. Bull. (Int. Ed.), 2014, 63, 2201 [Izv. Akad. Nauk, Ser. Khim., 2014, 2201].
E. V. Buravlev, I. Yu. Chukicheva, I. A. Dvornikova, A. V. Churakov, A. V. Kutchin, Russ. J. Org. Chem. (Engl. Transl.), 2012, 48, 938 [Zh. Org. Khim., 2012, 48, 943].
R. Munday, J. S. Munday, C. M. Munday, Free Radical Biol. Med., 2003, 34, 1200.
S. Henkelman, G. Rakhorst, J. Blanton, W. van Oeveren, Mat. Sci. Eng., C, 2009, 29, 1650.
J. Takebayashi, J. Chen, A. Tai, in Advanced Protocols in Oxidative Stress II, Methods in Molecular Biology, Ed. D. Armstrong, Humana Press, New York–Dordrecht–Heidelberg–London, 2010, 594, 287.
C. Wang, X. Qin, B. Huang, F. He, C. Zeng, Biochem. Biophys. Res. Commun., 2010, 402, 773.
P. S. C. Preté, C. C. Domingues, N. C. Meirelles, S. V. P. Malheiros, F. M. Goñi, E. Paula, S. Schreier, Biochim. Biophys. Acta, Biomembr., 2011, 1808, 164.
P. M. Rodi, V. M. Trucco, A. M. Gennaro, Biophys. Chem., 2008, 135, 14.
A. López-Revuelta, J. I. Sánchez-Gallego, A. HernándezHernández, J. Sánchez-Yagüe, M. Llanillo, Chem.-Biol. Interact., 2006, 161, 79.
M. Blasa, M. Candiracci, A. Accorsi, M. P. Piacentini, E. Piatti, Food Chem., 2007, 104, 1635.
S. Chaudhuri, A. Banerjee, K. Basu, B. Sengupta, P. K. Sengupta, Int. J. Biol. Macromol., 2007, 41, 42.
M. Suwalsky, P. Vargas, M. Avello, F. Villena, C. P. Sotomayor, Int. J. Pharm., 2008, 363, 85.
P. Filipe, A. M. S. Silva, R. S. G. R. Seixas, D. C. G. A. Pinto, A. Santos, L. K. Patterson, J. N. Silva, J. A. S. Cavaleiro, J. P. Freitas, J.-C. Mazière, R. Santus, P. Morlière, Biochem. Pharmacol., 2009, 77, 957.
Y. Chen, P. Deuster, Chem.-Biol. Interact., 2009, 182, 7.
C. L. Basiglio, E. J. S. Pozzi, A. D. Mottino, M. G. Roma, Chem.-Biol. Interact., 2009, 179, 297.
R. M. Costa, A. S. Magalhres, J. A. Pereira, P. B. Andrade, P. Valentão, M. Carvalho, B. M. Silva, Food Chem. Toxicol., 2009, 47, 860.
T. Asakawa, S. Matsushita, Lipids, 1980, 15, 137.
J. J. M. van den Berg, J. A. F. Op den Kamp, B. H. Lubin, B. Roelofsen, F. A. Kuypers. Free Radical Biol. Med., 1992, 12, 487.
Author information
Authors and Affiliations
Corresponding author
Additional information
On the occasion of the 100th anniversary of the birth of Academician N. K. Kochetkov (1915–2005).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1406–1412, June, 2015.
Rights and permissions
About this article
Cite this article
Buravlev, E.V., Chukicheva, I.Y., Sukrusheva, O.V. et al. Membrane protective properties of carboxy derivatives based on 2,6-diisobornyl-4-methylphenol. Russ Chem Bull 64, 1406–1412 (2015). https://doi.org/10.1007/s11172-015-1024-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-015-1024-1