Abstract
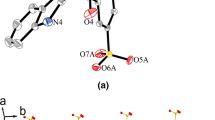
Novel ligands were synthesized by the reaction of benzazine-2-carbaldehydes or methyl pyrazinyl ketone with benzhydrazide, as well as their ZnII and CdII complexes with the N,N,O environment. Their structures and photophysical properties were studied. The synthesized complexes are photoluminescent.
Similar content being viewed by others
References
N.M. Shavaleev, R. Scopelliti, F. Gumy, J. C. G. Bunzli, Inorg. Chem., 2008, 47, 9055.
F. Rizza, A. Papagni, F. Meinardi, R. Tubino, M. Ottonelli, G. F. Musso, G. Dellepiane, Synth. Met., 2004, 147, 143.
S. Comby, D. Imbert, C. Vandevyver, J. C. G. Bunzli, Chem. Eur. J., 2007, 13, 936.
M. Albrecht, O. Osetska, R. Frohlich, J. C. G. Bunzli, A. Aebischer, F. Gumi, F. Hamacek, J. Am. Chem. Soc., 2007, 129, 14178.
M. Albrecht, S. Mirtschin, O. Osetska, S. Dehn, D. Enders, R. Frohlich, T. Pape, E.F. Hahn, Eur. J. Inorg. Chem., 2007, 3276.
T. Arslan, C. Ogretir, M. Tsiouri, J. C. Plakatouras, N. Hadjiliadis, J. Coord. Chem., 2007, 60, 699.
M. Albrecht, O. Osetska, J. Klankermayer, R. Frohlich, F. Gumy, J. C. G. Bunzli, Chem. Commun., 2007, 1834.
L. Tang, P. Zhou, Z. Qi, Z. Huang, J. Zhao, M. Cai, Bull. Korean Chem. Soc., 2013, 34, 2256.
Y. Liu, Z. Yang, J. Inorg. Biochem., 2009, 103, 1014.
Y. Liu, Z. Yang, J. Organomet. Chem., 2009, 694, 3091.
G. Maayan, Y. Dayagi, R. Arad-Yellin, L. J. W. Shimon, A. Shanzer, Polyhedron, 2013, 64, 365.
L. Tang, J. Zhao, M. Cai, P. Zhou, K. Zhong, S. Hou, Y. Bian, Tetrahedron Lett., 2013, 54, 6105.
S. Adsule, V. Barve, D. Chen, F. Ahmed, Q. P. Dou, S. Padhye, F. H. Sarkar, J. Med. Chem., 2006, 49, 7242.
T. V. Trashakhova, E. V. Nosova, P. A. Slepukhin, M. S. Valova, G. N. Lipunova, V. N. Charushin, Russ. Chem. Bull. (Int. Ed.), 2011, 60, 2347 [Izv. Akad. Nauk, Ser. Khim., 2011, 2302].
Z. C. Liao, Z. Y. Yang, Y. Li, B. D. Wang, Q. X. Zhou, Dyes Pigm., 2013, 97, 124.
Y. W. Shi, M. M. Shi, J. C. Huang, H. Z. Chen, M. Wang, M. D. Lin, Y. G. Ma, H. Xu, B. Yang, Chem. Commun., 2006, 1941.
Eksperimental’nye metody khimicheskoi kinetiki: Uchebnoe posobie [Experimental Methods of Chemical Kinetics: School-Book], Eds N. M. Emmanuel and M. G. Kuz’min. Izd-vo MGU, Moscow, 1985, p. 158–160 (in Russian).
G. M. Sheldrick, Acta Crystallogr. Sec A., 2008, 64, 112.
A. Tang, E. J. Lien, M. M. C. Lai, J. Med. Chem., 1985, 28, 1103.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences O. N. Chupakhin on the occasion of his 80th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1344–1349, June, 2014.
Rights and permissions
About this article
Cite this article
Nosova, E.V., Chupakhin, A.A., Lipunova, G.N. et al. Syntheses, structures, and photophysical properties of ZnII and CdII metal complexes based on benzoylhydrazones. Russ Chem Bull 63, 1344–1349 (2014). https://doi.org/10.1007/s11172-014-0601-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-014-0601-z