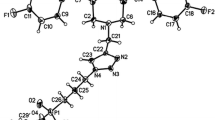
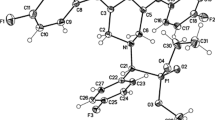
Abstract
An approach to the synthesis of new conjugates of 3,5-bis(arylidene)-4-piperidone pharmacophore with bisphosphonate moiety separated from the nitrogen atom of piperidone ring by four methylene units has been developed. This approach is based on the reaction of tetraethyl vinylidene-1,1-bisphosphonate with the Grignard reagent containing a dioxolane-protected piperidone ring (4-piperidone ethylene ketal), which is followed by the crotonic condensation of bisphosphonate obtained with aromatic aldehydes. In vitro evaluation of antitumor activity of prepared conjugates 3a,b toward human cancer cell lines Caov3, Scov3, KB3-1, KB8-5, A549, and MCF7 has revealed that these compounds possess moderate antitumor activity and are inferior in cytotoxicity to the analogs 1a,b with one methylene unit between piperidone and bisphosphonate moieties.
Similar content being viewed by others
References
U. Das, R. K. Sharma, J. R. Dimmock, Curr. Med. Chem., 2009, 16, 2001.
M. Gregory, A. Dandavati, M. Lee, S. Tzou, M. Savagian, K. A. Brien, V. Satam, P. Patil, M. Lee, Med. Chem. Res., 2013, 22, 5588.
T. K’alai, M. L. Kuppusamy, M. Balog, K. Selvendiran, B. K. Rivera, P. Kuppusamy, K. Hideg, J. Med. Chem., 2011, 54, 5414.
S. Das, U. Das, D. Michel, D. K. J. Gorecki, J. R. Dimmock, Eur. J. Med. Chem., 2013, 64, 321.
M. V. Makarov, E. S. Leonova, E. Yu. Rybalkina, V. N. Khrustalev, N. E. Shepel, G.-V. Röschenthaler, T. V. Timofeeva, I. L. Odinets, Arch. Pharm. Chem. Life Sci., 2012, 345, 349.
F. R. Quinn, G. W. A. Milne, Fundam. Appl. Toxicol., 1986, 6, 270.
M. V. Makarov, E. S. Leonova, E. Yu. Rybalkina, P. Tongwa, V. N. Khrustalev, T. V. Timofeeva, I. L. Odinets, Eur. J. Med. Chem., 2010, 45, 992.
E. S. Leonova, M. V. Makarov, E. Yu. Rybalkina, S. L. Nayani, P. Tongwa, A. Fonari, T. V. Timofeeva, I. L. Odinets, Eur. J. Med. Chem., 2010, 45, 5926.
A. E. Shipov, M. V. Makarov, P. V. Petrovskii, E. Yu. Rybalkina, Yu. V. Nelyubina, I. L. Odinets, Heteroatom Chem., 2013, 24, 191.
M. V. Makarov, E. Yu. Rybalkina, G.-V. Röschenthaler, K. W. Short, T. V. Timofeeva, I. L. Odinets, Eur. J. Med. Chem., 2009, 44, 2135.
R. G. G. Russell, Phosphorus, Sulfur, Silicon, Relat. Elem. 1999, 144–146, 793.
K. Stach, M. Thiel, F. Bickelhaupt, Monatshefte für Chemie, 1962, 93, 1090.
M. L. Lolli, L. Lazzarato, A. Di Stilo, R. Fruttero, A. Gasco, J. Organomet. Chem., 2002, 650, 77.
J. van Meerloo, G. J. L. Kaspers, J. Cloos, in Methods in Molecular Biology, Ed. I. A. Cree, Humana Press, 2011, 731, 502.
C. R. Degenhardt, D. C. Burdsall, J. Org. Chem., 1986, 51, 3488.
Author information
Authors and Affiliations
Corresponding author
Additional information
Based on the Materials of the First Russian Conference on Medicinal Chemistry (MedChem Russia-2013) with International Participation (September 8–12, 2013, Moscow).
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 1181–1186, May, 2014.
Rights and permissions
About this article
Cite this article
Makarov, M.V., Rybalkina, E.Y. & Röschenthaler, G.V. New 3,5-bis(arylidene)-4-piperidones with bisphosphonate moiety: synthesis and antitumor activity. Russ Chem Bull 63, 1181–1186 (2014). https://doi.org/10.1007/s11172-014-0569-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-014-0569-8