Abstract
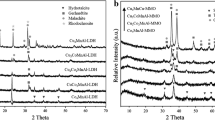
Influence of the nature of a doubly charged cation in the layered double hydroxides (LDH) on the conditions of formation and properties of mixed oxide phase MAlO x (M = Mg2+, Zn2+ and Ni2+), its ability to reconstruct the structure of the original precursor under contact with water has been studied. Hydrotalcite-like compounds and corresponding oxides with different M2+: M3+ ratio were investigated by XRD, TEM, TG-DTG-DTA, 27Al NMR, N2 adsorption, and differentiating dissolution. It has been found that the nature of the cation M2+ influences the conditions of LDH thermal decomposition, structural and textural characteristics of the formed mixed oxides. The obtained data can be used to synthesize the oxide supports with desired acid-base and adsorption properties.
Similar content being viewed by others
References
S. Cavallaro, V. Chiodo, S. Freni, N. Mondello, F. Frusteri, Appl. Catal. A: General, 2003, 249, 119.
D. K. Liguras, D. I. Kondarides, X. E. Verykios, Appl. Catal. B: Environmental, 2003, 43, 345.
F. Marico, M. Jobbagy, G. Baronetti, Laborde, M., in Studies in Surface Science and Catalysis, Eds F. V. M. S. M. Avelino Corma, G.F. José Luis, 2000, Elsevier, p. 2147.
F. Marico, Appl. Catal. A: General, 2003, 238, 41.
J. Llorca, J. Catal., 2002, 209, 306.
J. Comas, F. Marico, M. Laborde, N. Amadeo, Chem. Eng. J., 2004, 98, 61.
D. Tichit, F. Medina, B. Coq, R. Dutartre, Appl. Catal. A: General, 1997, 159, 241.
G. J. Soler-Iltia, M. Jobbagy, R. Candal, A. E. Regazzoni, M. A. Blesa, J. Disp. Sci. Technol., 1998, 19, 207.
T. Shishido, Y. Yamamoto, H. Morioka, K. Takaki, K. Takehira, Appl. Catal. A: General, 2004, 263, 249.
F. Marico, M. Boveri, G. Baronetti, M. Laborde, Int. J. Hydrogen Energy, 2004, 29, 67.
V. Rives, M. A. Ulibary, Coord. Chem. Rev., 1999, 181, 61.
D. G. Evance, R. C. T. Slade, in Layered Double Hydroxides, Springer-Verlag Heidelberg, Berlin, Germany, 2006, p. 1.
O. B. Bel’skaya, N. N. Leontéva, T. I. Gulyaeva, V. A. Drozdov, V. P. Doronin, V. I. Zaikovsky, V. A. Likholobov, Kinetica i kataliz, 2011, 52, 778 [Kinet. Catal. (Engl. Transl.), 2011, 52].
O. B. Bel’skaya, N. N. Leontéva, T. I. Gulyaeva, V. A. Drozdov, V. P. Doronin, V. I. Zaikovsky, V. A. Likholobov, Kinetica i kataliz, 2011, 52, 899 [Kinet. Catal. (Engl. Transl.), 2011, 52].
F. Cavani, F. Trifirb, A. Vaccari, Catal. Today, 1991, 11, 173.
X. Wang, Q. Zhang, Polymer Int. J., 2004, 53, 698.
G. Camino, A. Maffezzoli, M. Braglia, M. De Lazzaro, M. Zammarano, Polymer Degrad. Stab., 2001, 74, 457.
A. Ookubo, K. Ooi, H. Hayashi, J. Pharm. Sci., 1992, 81, 1139.
M. Del Arco, E. Cebadera, S. Gutiérrez, C. Martín, M. J. Montero, V. Rives, J. Rocha, M. A. Sevilla, J. Pharm. Sci., 2004, 93, 1649.
C. Resini, T. Montanari, L. Barattini, G. Ramis, G. Busca, S. Presto, P. Riani, R. Marazza, M. Sisani, F. Marmottini, U. Costantino, Appl. Catal. A: General, 2009, 355, 83.
E. C. Kruissink, H. L. Pelt, J. R. H. Ross, L. L. Van Reüen, Appl. Catal., 1981, 1, 23.
L. Bournay, D. Casanave, B. Delfort, G. Hillion, J. A. Chodorge, Catal. Today, 2005, 106, 190.
W. Breuer, H. C. Raths, US patent to Henkel, 1994.
G. Wolf, pat. US 5741947 to BASF, 1998.
B. M. Welch, pat. US 4620053 to Phillips Petroleum Company, 1986.
M. V. Twigg, Catalyst Handbook, 2nd ed., Wolfe Pub, London, 1989.
M. Turco, G. Bagnasco, U. Costantino, F. Marmottini, T. Montanari, G. Ramis, G. Busca, J. Catalysis, 2004, 228, 43.
T. Montanari, M. Sisani, M. Nocchetti, R. Vivani, M. Delgado, C. Herrera, G. Ramis, G. Busca, U. Costantino, Catal. Today, 2010, 152, 104.
C. Yu, H. Xu, Q. Ge, W. Li, J. Mol. Catal. A: Chemical, 2007, 266, 80.
P. Beaudot, M. E. De Roy, J. P. Besse, J. Solid St. Chem., 2004, 177, 2691.
Y. Wang, S. Wang, X. Guo, S.-M. Zhang, W.-P. Huang, Sh. Wu, Catal. Letters, 2009, 132, 472.
A. Bhattacharyya, V. W. Chang, D. J. Schumacher, Appl. Clay Sci., 1998, 13, 317.
K. O. Christensen, D. Chen, R. Lødeng, A. Holmen, Appl. Catal. A: General, 2006, 314, 9.
P. Cardoso, L. J. B. Valim, J. Phys. Chem. Solids, 2004, 65, 481.
H. Nakayama, N. Wada, M. Tsuhako, Int. J. Pharm., 2004, 269, 469.
K. K. Rao, M. Gravelle, J. S. Valente, F. Figueras, J. Catalysis, 1998, 173, 115.
J. C. A. A. Roelofs, D. J. Lensveld, A. J. van Dillen, K. P. de Jong J. Catalysis, 2001, 203, 184.
S. Abelly, F. Medina, D. Tichit, J. Pérez-Ramírez, J. C. Groen, J. E. Sueiras, P. Salagre, Y. Cesteros, Chem. Eur. J., 2005, 11, 728.
T. Sato, H. Fujita, T. Endo, M. Shimada, A. Tsunashima, Reactivity of Solids, 1988, 5, 219.
O. Clause, B. Rebours, E. Merlen, F. Trifiry, A. Vaccari, J. Catal., 1992, 133, 231.
J. Pérez-Ramírez, Materials Research Bulletin, 2001, 36, 1767.
C. Forano, T. Hibino, F. Leroux, C. Taviot-Gue’ho, in Handbook of Clay Science, Ed. G. L. F. Bergaya, Elsevier Ltd., 2006, 1021.
E. P. Barrett, L. G. Joiner, P. H. Halenda, J. Am. Chem. Soc., 1951, 73, 373.
V. V. Malakhov, Zhurn. analit. khimii, 1989, 44, 1177 [J. Anal. Chem. (Engl. Transl.), 1989, 44].
V. V. Malakhov, Dokl. AN SSSR, 1986, 290, 1152 [Dokl. Chem. (Engl. Transl.), 1986, 290].
A. S. Shaporev, Dokl. AN, 2009, 426, 194 [Dokl. Chem. (Engl. Transl.), 2009, 426].
A. S. Bookin, V. A. Drits, Clays and Clay Minerals, 1993, 41, 551.
N. N. Leontéva, S. V. Cherepanova, O. B. Bel’skaya, V. A. Drozdov, V. P. Talzi, Chimiya v interesach ustoichivogo razvitiya, 2013, 21, 69.
J. Pérez-Ramírez, S. Abelló, N. M. van der Pers, J. Phys. Chem. C, 2007, 111, 3642.
R. D. Shannon, Acta Crystallogr., Sect. A, 1976, 32, 751.
E. Lopez-Salinas, E. Torres-Garcia, M. Garcia-Sanchez, J. Phys. Chem. Solids, 1997, 58, 919.
W. Yang, Y. Kim, P. K. T. Liu, M. Sahimi, Th. T. Tsotsis, Chem. Eng. Sci., 2002, 57, 2945.
S. R. Segal, K. B. Anderson, K. A. Carrado, C. L. Marshall, Appl. Catal. A: General, 2002, 231, 215.
D. Kishore, S. Kannan, Appl. Catal. A: General, 2004, 270, 227.
E. Kanezaki, Inorg. Chem., 1998, 37, 2588.
T. Stanimirova, I. Vergilov, G. Kirov, N. Petrova, J. Mater. Sci., 1999, 34, 4153.
J. S. Valente, F. Figueras, M. Gravelle, P. Kumbhar, J. Lopez, J. P. Besse, J. Catalysis, 2000, 189, 370.
ICDD, PDF-2.
S. Velu, V. Ramkumar, A. Narayanan, C. S. Swamy, J. Mater. Sci., 1997, 32, 957.
M. R. Weir, R. A. Kydd, Inorg. Chem., 1998, 37, 5619.
C. Depège, F.-Z. El Metoui, C. Forano, A. de Roy, J. Dupuis, J.-P. Besse, Chemistry of Materials, 1996, 8, 952.
E. Kanezaki, J. Incl. Phen. Macr. Chem., 2003, 46, 89.
M. Jitianu, A. Jitianu, M. Zaharescu, D. Crisan, R. Marchidan, Vibrational Spectroscopy, 2000, 22, 75.
L. Smoláková, L. Èapek, Š. Botková, F. Kovanda, R. Bulánek, M. Pouzar, Topics in Catalysis, 2011, 54, 1151.
L. E. Alzamora, J. R. H. Ross, E. C. Kruissink, L. L. van Reijen, J. Chem. Soc., Faraday Trans. 1, 1981, 77, 665.
O. Clause, M. Goncalves Coelho, M. Gazzano, D. Matteuzzi, F. Trifirò, A. Vaccari, Appl. Clay Sci., 1993, 8, 169.
B. Rebours, J.-B. dÉspinose de la Caillerie, O. Clause, J. Am. Chem. Soc., 1994, 116, 1707.
S. Abelló, D. Verboekend, B. Bridier, J. Pérez-Ramírez, J. Catal., 2008, 259, 85.
O. Lebedeva, D. Tichit, B. Coq, Appl. Catal. A: General, 1999, 183, 61.
R. V. Prikhod’ko, M. V. Sychev, I. M. Astrelin, K. Erdmann, A. Mangel, R. A. van Santen, Russ. J. Appl. Chem. (Engl. Transl.), 2001, 74, 1621.
N. N. Leont’eva, S. V. Cherepanova, O. B. Bel’skaya, V. A. Drozdov, V. P. Talzi, Teoret. eksperim. khimiya, 2012, 48, 257 [Theor. Exp. Chem. (Engl. Transl.), 2012, 48].
A. S. Ivanovs, G. S. Litvak, G. N. Kryukova, S. V. Tsybulya, E. A. Paukshtis, Kinetika i kataliz, 2000, 41, 137 [Kinet. Catal. (Engl. Transl.), 2000, 41].
D. Meloni, R. Monaci, V. Solinas, A. Auroux, E. Dumitriu, Appl. Catal. A: General, 2008, 350, 86.
M. J. Holgado, V. Rives, M. S. San Román, Appl. Catal. A: General, 2001, 214, 219.
L. Pesic, S. Salipurovic, V. Markovic, D. Vucelic, W. Kagunya, W. Jones, J. Mater. Chem., 1992, 2, 1069.
J. P. Franck, E. Freund, E. Quemere, J. Chem. Soc., Chem. Commun., 1984, 10, 629.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences M. P. Egorov on the occasion of his 60th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 2349–2361, Novemer, 2013.
Rights and permissions
About this article
Cite this article
Belskaya, O.B., Leont’eva, N.N., Gulyaeva, T.I. et al. Influence of a doubly charged cation nature on the formation and properties of mixed oxides MAlO x (M = Mg2+, Zn2+, Ni2+) obtained from the layered hydroxide precursors. Russ Chem Bull 62, 2349–2361 (2013). https://doi.org/10.1007/s11172-013-0341-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-013-0341-5