Abstract
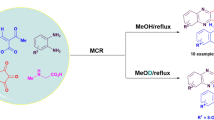
Diazotization of vicinal 1-amino-2-ethynyl-4-R-9,10-anthraquinones followed by a reaction with NaN3 gave 5-hydroxy-3-R-1H-naphtho[2,3-g]indazole-6,11-diones or 3-ethynyl-5-R-6H-anthra[1,9-cd]isoxazol-6-ones, depending on the substituents at the triply bonded C atom and in position 4 of the anthraquinone framework.
Similar content being viewed by others
References
V. M. Dembitsky, D. O. Levitsky, Nat. Prod. Commun., 2006, 1, 405.
U. Galm, M. H. Hager, S. G. Van Lanen, J. Ju, J. S. Thorson, B. Shen, Chem. Rev., 2005, 105, 739.
F. Diederich, P. J. Stang, R. R. Tykwinski, Acetylene Chemistry: Chemistry, Biology and Material Science, Wiley-VCH, Weinheim, 2005, 508 pp.
T. Tuttle, E. Kraka, D. J. Cremer, J. Am. Chem. Soc., 2005, 127, 9469.
J. Davies, H. Wang, T. Taylor, K. Warabi, X.-H. Huang, R. J. Andersen, Org. Lett., 2005, 7, 5233.
A. G. Myers, S. B. Cohen, N. J. Tom, D. J. Madar, M. E. Fraley, J. Am. Chem. Soc., 1995, 117, 7574.
S. Vasilevsky, L. Gornostaev, A. Stepanov, E. Arnold, I. Alabugin, Tetrahedron Lett., 2007, 48, 1867.
A. A. Stepanov, L. M. Gornostaev, S. F. Vasilevsky, E. V. Arnold, V. I. Mamatyuk, D. S. Fadeev, B. Gold, I. V. Alabugin, J. Org. Chem., 2011, 76, 8737.
M. S. Sokolova, V. A. Beresnev, O. I. Kargina, L. M. Gornostaev, Zh. Org. Khim., 2008, 44, 1654 [Russ. J. Org. Chem. (Engl. Transl.), 2008, 44].
M. S. Shvartsberg, I. D. Ivanchikova, L. G. Fedenok, Tetrahedron Lett., 1994, 35, 6749.
D. N. Laikov, Yu. A. Ustynyuk, Russ. Chem. Bull. (Int. Ed.), 2005, 54, 820 [Izv. Akad. Nauk, Ser. Khim., 2005, 804].
D. N. Laikov, Chem. Phys. Lett., 1997, 281, 151.
D. N. Laikov, Chem. Phys. Lett., 2005, 416, 116.
J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865.
D. E. Ames, D. Bull, C. Takundwa, Synthesis, 1981, 364.
V. T. Sakilidi, L. M. Gornostaev, Zh. Org. Khim., 1982, 18, 1084 [J. Org. Chem. USSR (Engl. Transl.), 1982, 18].
L. M. Gornostaev, G. F. Zeibert, G. I. Zolotareva, Khim. Geterotsikl. Soedin., 1980, 912 [Chem. Heterocycl. Compd. (Engl. Transl.), 1980, 7].
L. F. Tietze, J. Görlitzer, A. Schuffenhauer, M. Hübner, Eur. J. Org. Chem., 1999, 1075.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 2071–2078, November, 2012.
Rights and permissions
About this article
Cite this article
Vasilevsky, S.F., Stepanov, A.A. & Fadeev, D.S. Dual reactivity of diazonium salts derived from 1-amino-2-ethynyl-9,10-anthraquinones. Russ Chem Bull 61, 2088–2095 (2012). https://doi.org/10.1007/s11172-012-0292-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-012-0292-2