Abstract
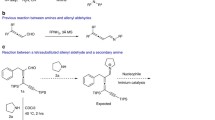
(2E,4E)-2-(2-Benzyloxyethyl)-5-(4-chloro-3-methoxyphenyl)penta-2,4-dienal was obtained by the condensation of 4-benzyloxybutanal N-tert-butylimine with 4-chloro-3-methoxycinnamic aldehyde with ≥98% configurational purity and 40% yield. When 4-benzyloxy-2-triethylsilylbutanal imine was used, a 7: 3 mixture of the target (2E,4E)-dienal with its (2Z,4E)-isomer was obtained in 60% yield; the latter quantitatively isomerized to the thermodynamically preferable target (2E,4E)-dienal.
Similar content being viewed by others
References
N. Ya. Grigorieva, A. G. Smirnov, V. A. Popovsky, A. V. Stepanov, Mendeleev Commun., 2008, 18, 84.
J. M. Clough, Nat. Prod. Rep., 1993, 10, 565.
H. Sauter, W. Steglich, T. Anke, Angew. Chem., Int. Ed., 1999, 38, 1329.
N. Ya. Grigorieva, V. A. Popovskii, A. V. Stepanov, E. D. Lubuzh, Izv. Akad. Nauk, Ser. Khim., 2010, 2033 [Russ. Chem. Bull., Int. Ed., 2010, 59, 2086].
K. Beautement, J. M. Clough, P. J. de Fraine, Ch. R. A. Godfrey, Pestic. Sci., 1991, 31, 499.
H. Sutter, Tetrahedron Lett., 1989, 30, 5417.
N. Ya. Grigorieva, A. G. Smirnov, V. A. Popovsky, A. V. Stepanov, Russ. Chem. Bull. (Int. Ed.), 2009, 58, 312 [Izv. Akad. Nauk, Ser. Khim., 2009, 310].
F. Claudi, G. Giorgioni, A. D. Stefano, M. P. Abbracchio, A. M. Paoletti, W. Baduini, J. Med. Chem., 1992, 35, 4408.
I. N. Nazarov, S. M. Makin, Zh. Obshch. Khim., 1959, 29, 3685 [J. Gen. Chem. USSR (Engl. Transl.), 1959, 29]; S. M. Makin, G. A. Ermakova, O. A. Shavrygina, A. P. Pleshakova, V. N. Voznesenskii, Zh. Org. Khim., 1979, 15, 1852 [J. Org. Chem. USSR (Engl. Transl.), 1979, 15].
N. Ya. Grigorieva, O. N. Yudina, O. A. Pinsker, E.D. Daeva, A. M. Moiseenkov, Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.), 1990, 39, 84 [Izv. Akad. Nauk SSSR, Ser. Khim., 1990, 97].
I. M. Avrutov, N. Ya. Grigorieva, V. S. Bogdanov, A. M. Moiseenkov, Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.), 1987, 36, 2033 [Izv. Akad. Nauk SSSR, Ser. Khim., 1987, 2194].
R. Baudouy, Ph. Prince, Tetrahedron, 1989, 45, 2067.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1599–1604, August, 2012.
Rights and permissions
About this article
Cite this article
Popovsky, V.A., Stepanov, A.V. & Grigorieva, N.Y. Preparation of (2E,4E)-2-(2-benzyloxyethyl)-5-(3-methoxy-4-chlorophenyl)penta-2,4-dienal as a key intermediate in the synthesis of strobilurin B. Russ Chem Bull 61, 1616–1621 (2012). https://doi.org/10.1007/s11172-012-0215-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-012-0215-2