Abstract
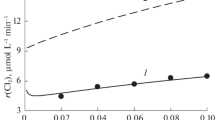
Nitroxyl radical TEMPO is found to catalyze the decomposition of sodium hypochlorite in an aqueous-alkali medium. The mechanism of NaOCl decomposition to form ClO· radical is proposed which involves protonated TEMPO, oxoammonium salt TEMPO+, and hydroxyl radical.
Similar content being viewed by others
References
A. E. J. de Nooy, A. C. Besemer, H. van Bekkum, Synthesis, 1996, 10, 1153.
I. Yu. Ponedel’kina, E. A. Khaibrakhmanova, V. N. Odinokov, Usp. Khim., 2010, 79, 65 [Russ. Chem. Rev. (Engl. Transl.), 2010, 79, 63].
P. L. Bragd, H. van Bekkum, A. C. Besemer, Top. Catal., 2004, 27, 49.
A. E. J. de Nooy, A. C. Besemer, H. van Bekkum, Carbohydr. Res., 1995, 269, 89.
A. Jiang, E. Drouet, M. Milas, M. Rinaudo, Carbohydr. Res., 2000, 327, 455.
Y. Kato, R. Matsuo, A. Isogai, Carbohydr. Polym., 2003, 69, 51.
M. H. Hashmi, A. A. Ayaz, A. Rashid, E. Ali, Anal. Chem., 1964, 36, 1379.
D. M. Stanbury, Adv. Inorg. Chem., 1989, 33, 69.
J. R. Fish, S. G. Swarts, M. D. Sevilla, T. Malinski, J. Phys. Chem., 1988, 92, 3745.
L. C. Adam, I. Fabian, K. Suzuki, G. Gordon, Inorg. Chem., 1992, 31, 3534.
L. C. Adam, G. Gordon, Inorg. Chem., 1999, 38, 1299.
B. P. Nikol’skii, V. G. Krunchak, T. V. L’vova, V. V. Pal’chevskii, R. I. Sosnovskii, Dokl. Akad. Nauk, 1970, 191, 140 [Dokl. Chem. (Engl. Transl.), 1970].
B. P. Nikol’skii, V. G. Krunchak, V. V. Pal’chevskii, R. I. Sosnovskii, Dokl. Akad. Nauk, 1971, 197, 1324 [Dokl. Chem. (Engl. Transl.), 1971].
A. U. Khan, M. Kasha, Proc. Natl. Acad. Sci. USA, 1994, 91, 12362.
J. A. Cella, J. A. Kelley, E. F. Kenehan, J. Org. Chem., 1975, 40, 1860.
P. L. Anelli, C. Biffi, F. Montanari, S. Quici, J. Org. Chem., 1987, 52, 2559.
T. Miyazawa, T. Endo, J. Org. Chem., 1985, 50, 3930.
J. C. Morris, J. Phys. Chem., 1966, 70, 3798.
J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865.
D. N. Laikov, Chem. Phys. Lett., 2005, 416, 116.
D. N. Laikov, Yu. A. Ustynyuk, Izv. Akad. Nauk, Ser. Khim., 2005, 804 [Russ. Chem. Bull., Int. Ed., 2005, 54, 820].
D. Sh. Sabirov, R. G. Bulgakov, Comput. Theor. Chem., 2011, 963, 185.
A. F. Shestakov, Ross. Khim. Zh., 2007, 51, 121 [Mendeleev Chem. J. (Engl. Transl.), 2007, 51].
F. Emmenegger, G. Gordon, Inorg. Chem., 1967, 6, 633.
H. Taube, H. Dodgen, J. Am. Chem. Soc., 1949, 71, 3330.
G. V. Buxton, M. S. Sumhani, J. Chem. Soc., Faraday Trans., 1972, 68, 947.
G. Porter, F. J. Wright, Disc. Faraday Soc., 1953, 14, 23.
R. A. Cox, R. G. Derwent, A. E. Eggleton, H. J. Reid, J. Chem. Soc., Faraday Trans., 1979, 75, 1648.
R. A. Durie, D. A. Ramsay, Canad. J. Phys., 1958, 36, 35.
E. G. Rozantsev, V. D. Sholle, Synthesis, 1971, 401.
M. Dagonneau, E. S. Kagan, V. I. Mikhailov, E. G. Rozantsev, V. D. Sholle, Synthesis, 1984, 895.
T. Endo, T. Miyazawa, S. Shiihashi, M. Okawara, J. Am. Chem. Soc., 1984, 106, 3877.
Z. B. Alfassi, R. E. Huie, S. Mosseri, P. Neta, Radiat. Phys. Chem., 1988, 43, 85.
G. V. Buxton, M. S. Sumhani, J. Chem. Soc., Faraday Trans., 1972, 68, 958.
J. A. Epstein, M. Lewin, J. Polym. Sci., 1962, 58, 991.
O. Iguerb, S. Demoustier-Champagne, J. Marchand-Brynaert, D. Daoust, M. Sclavons, J. Devaux, J. Appl. Polym. Sci., 2006, 100, 1184.
G. V. Buxton, F. S. Dainton, F. Wilkinson, Chem. Commun., 1966, 320.
L. Wang, D. W. Margerum, Inorg. Chem., 2002, 41, 6099.
V. Csordas, B. Bubnis, I. Fabian, G. Gordon, Inorg. Chem., 2001, 40, 1833.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1164–1169, June, 2012.
Rights and permissions
About this article
Cite this article
Ponedel’kina, I.Y., Khaibrakhmanova, E.A., Sabirov, D.S. et al. Decomposition of sodium hypochlorite in an aqueous solution in the presence of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) nitroxyl radical as a catalyst. Russ Chem Bull 61, 1176–1181 (2012). https://doi.org/10.1007/s11172-012-0160-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-012-0160-0