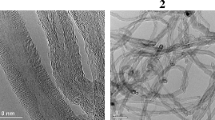
Abstract
An electrochemical method for dispersion of single-walled carbon nanotubes (SWNTs) is described. The technique is based on grafting of oxygen-containing functional groups to the nanotube surface during electrolysis in aqueous and nonaqueous potassium bromide solutions. A dependence of the degree of functionalization of nanotubes on the solvent was revealed experimentally. Nanotubes treated in DMSO have about 14 carbon atoms per oxygen atom from functional groups (cf. nearly four C atoms per oxygen atom in the nanotubes treated in aqueous solutions). The corresponding maximum specific capacities of the electrodes are nearly 10 and 60 F g−1. The samples treated in solutions of KBr in DMSO have about 300 carbon atoms per bromine atom on the nanotube surface (cf. only 30 carbon atoms in the samples treated in aqueous solution). A mechanism of electrochemical modification of SWNTs is proposed. Its key step is production of atomic oxygen that oxidizes the nanotube surface with the formation of functional groups.
Similar content being viewed by others
References
B. S. Banerjee, T. Hemraj-Benny, Adv. Mater., 2005, 17, 17.
E. R. Badamshina, M. P. Gafurova, Ya. I. Estrin, Usp. Khim., 2010, 79, 1027 [Russ. Chem. Rev. (Engl.Trans.), 2010, 79, 945].
M. Moniruzzaman, K. I. Winey, Macromolecules, 2006, 39, 5194.
P. M. Ajayan, J. M. Tour, Nature (London), 2007, 447, 1066.
V. G. Udovitsky, Fiz. Inzh. Poverkhn., 2009, 7, 351 [Physical Surface Engineering (Engl. Transl.), 2009, 7, No. 4].
L. Vaisman, H. D. Wagner, G. Marom, Adv. Colloid Interface Sci., 2006, 128–130, 37.
O. Tanaike, O. Kimizuka, N. Yoshizawa, K. Yamada, X. Wang, H. Hatori, M. Toyoda, Electrochem. Commun., 2009, 4, 1441.
P. M. Rafailov, C. Thomsen, U. Dettlaff-Weglikowska, S. Roth, J. Phys. Chem. B, 2008, 11, 25368.
C. Nieto-Draghi, J. B. Avalosa, J. Chem. Phys., 2003, 119, 4782.
V. V. Chaban, O. N. Kalugin, J. Mol. Liq., 2010, 151, 113.
S. Banerjee, S. S. Wong, J. Am. Chem. Soc., 2002, 124, 8940.
M. Pavese, S. Musso, S. Bianco, M. Giorcelli, N. Pugno, J. Phys. Condens. Matter, 2008, 20, 474206.
A. V. Krestinin, A. P. Haritonov, Yu. M. Shulga, O. M. Zhigalina, E. I. Knerelman, M. Dubois, M. M. Brzhezinskaya, A. S. Vinogradov, A. B. Preobrazhencky, G. I. Zvereva, M. B. Kislov, V. M. Martynenko, I. I. Korobov, G. I. Davydova, V. G. Zhigalina, N. A. Kiselev, Ros. nano-tekhnologii, 2009, 4, 67 [Nanotechnologies in Russia (Engl. Transl.), 2009, 4, 60].
A. G. Krivenko, V. I. Matyushenko, E. V. Stenina, L. N. Sviridova, A. V. Krestinin, G. I. Zvereva, V. A. Kurmaz, A. G. Ryabenko, S. N. Dmitriev, V. A. Skuratov, Electrochem. Commun., 2005, 7, 199.
K. Mizur, S. Imafuji, T. Ochi, J. Phys. Chem. B, 2000, 104, 11001.
M. C. R. Symons, G. Eaton, J. Chem. Soc., Faraday Trans. 1, 1985, 81, 1963.
Yu. A. Kukk, Zh. Klavil’ye, Elektrokhimiya, 1977, 13, 841 [Sov. Electrochemistry (Engl.Trans.), 1977, 13, 712].
A. P. Dementjeva, K. I. Maslakova, A. V. Naumkin, Appl. Surf. Sci., 2005, 245, 128.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1046–1052, June, 2011.
Rights and permissions
About this article
Cite this article
Krivenko, A.G., Komarova, N.S., Ryabenko, A.G. et al. The role of the medium in electrochemical functionalization and dispersion of carbon nanotubes. Russ Chem Bull 60, 1071–1077 (2011). https://doi.org/10.1007/s11172-011-0169-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-011-0169-9