Abstract
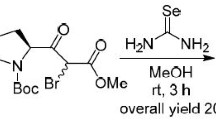
Nucleophilic substitution of the halogen atom in dimethyl (S)-4-bromoglutamate followed by removal of the protecting groups and closure of a lactam ring afforded (2S,4S)-4-(indolin-1-yl)-5-oxoproline. The indoline fragment was oxidized into the indole fragment to give (2S,4S)-4-(indol-1-yl)-5-oxoproline; reduction of the carbonyl groups with BH3 yielded (2S,4S)-4-(indol-1-yl)prolines and (2S,4S)-2-hydroxymethyl-4-(indol-1-yl)pyrrolidines. Reduction of (2S,4S)-4-arylamino-5-oxoprolines with BH3 to the corresponding (2S,4S)-4-arylaminoprolines and (2S,4S)-4-arylamino-2-hydroxymethylpyrrolidines was studied.
Similar content being viewed by others
References
H.-D. Jakubke, H. Jeschkeit, Aminosäuren, Peptide, Proteine, Eine Einführung, Akademie Verlag, Berlin, 1982, 505 S.
C. Nájera, M. Yus, Tetrahedron: Asymmetry, 1999, 10, 2245.
S. K. Panday, J. Prasad, D. K. Dikshit, Tetrahedron: Asymmetry, 2009, 20, 1581.
C. E. Schafmeister, Z. Z. Brown, S. Gupta, Acc. Chem. Res., 2008, 41, 1387.
R. B. Silverman, Acc. Chem. Res., 2009, 42, 439.
T. E. Kristensen, T. Hansen, Eur. J. Org. Chem., 2010, 3179.
S. G. Zlotin, A. S. Kucherenko, I. P. Beletskaya, Usp. Khim., 2009, 78, 796 [Russ. Chem. Rev. (Engl. Transl.), 2009, 78, 737].
A. ErkkilChem. Rev., 2007, 107, 5416.
S. P. Gupta, D. Kumar, S. Kumaran, Bioorg. Med. Chem., 2003, 11, 1975.
H. Sakashita, F. Akahoshi, H. Kitajima, R. Tsutsumiuchi, Y. Hayashi, Bioorg. Med. Chem., 2006, 14, 3662.
D. Torino, A. Mollica, F. Pinnen, F. Feliciani, S. Spisani, G. Lucente, Bioorg. Med. Chem., 2009, 17, 251.
S. Flemer, A. Wurthmann, A. Mamai, J. S. Madalengoitia, J. Org. Chem., 2008, 73, 7593.
A. Flores-Ortega, J. Casanovas, X. Assfeld, C. Aleman, J. Org. Chem., 2009, 74, 3101.
A. Evidente, A. Andolfi, M. Vurro, M. C. Zonno, A. Motta, Phytochemistry, 2000, 53, 231.
D. D. Schoepp, B. G. Johnson, C. R. Salhoff, M. J. Valli, M. A. Desai, J. P. Burnett, N. G. Mayne, J. A. Monn, Neuropharmacology, 1995, 34, 843.
M. G. Moloney, Nat. Prod. Rep., 2002, 19, 597.
V. Bruno, G. Battaglia, A. Copani, M. D’Onofrio, P. Di Iorio, A. De Blasi, D. Melchiorri, P. J. Flor, F. Nicoletti, J. Cereb. Blood Flow Metab., 2001, 21, 1013.
S. Süzen, Top. Heterocycl. Chem., 2007, 11, 145.
M. N. Preobrazhenskaya, A. M. Korolev, Bioorg. Khim., 2000, 26, 97 [Russ. J. Bioorg. Chem. (Engl. Transl.), 2000, 26, 85].
G. Laconde, P. Carato, J. H. Poupaert, P. Berthelot, P. Depreux, J.-P. Hénichart, Monatsh. Chem., 2003, 134, 1037 (and references cited therein).
V. P. Krasnov, I. M. Bukrina, E. A. Zhdanova, M. I. Kodess, M. A. Korolyova, Synthesis, 1994, 961.
V. P. Krasnov, A. Yu. Vigorov, I. A. Nizova, T. V. Matveeva, A. N. Grishakov, I. V. Bazhov, A. A. Tumashov, M. A. Ezhikova, M. I. Kodess, Eur. J. Org. Chem., 2007, 4257.
V. P. Krasnov, A. Yu. Vigorov, I. A. Nizova, A. N. Grishakov, N. G. Evstigneeva, M. I. Kodess, Izv. Akad. Nauk, Ser. Khim., 2004, 1274 [Russ. Chem. Bull., Int. Ed., 2004, 53, 1327].
I. A. Nizova, V. P. Krasnov, O. V. Korotovskikh, L. V. Alekseeva, Izv. Akad. Nauk SSSR, Ser. Khim., 1989, 2781 [Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.), 1989, 38, 2545].
M. N. Preobrazhenskaya, Usp. Khim., 1967, 36, 1760 [Russ. Chem. Rev. (Engl. Transl.), 1967, 36, 753].
C. J. Salomon, E. G. Mata, O. A. Mascaretti, J. Org. Chem., 1994, 59, 7259.
F. Lenda, F. Guenoun, J. Martinez, F. Lamaty, Tetrahedron Lett., 2007, 48, 805.
D. K. Dikshit, S. K. Panday, J. Org. Chem., 1992, 57, 1920.
V. P. Krasnov, I. A. Nizova, A. Yu. Vigorov, T. V. Matveeva, G. L. Levit, P. A. Slepukhin, M. A. Ezhikova, M. I. Kodess, Eur. J. Org. Chem., 2008, 1802.
J. Ezquerra, C. Pedregal, A. Rubio, B. Yruretagoyena, A. Escribano, F. Sanchez-Ferrando, Tetrahedron, 1993, 49, 8665.
E. A. Elin, V. V. Onoprienko, I. A. Kudelina, A. I. Miroshnikov, Bioorg. Khim., 2000, 26, 862 [Russ. J. Bioorg. Chem. (Engl. Transl.), 2000, 26, 774].
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician V. N. Charushin on the occasion of his 60th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 853–861, May, 2011.
Rights and permissions
About this article
Cite this article
Vigorov, A.Y., Nizova, I.A., Shalunova, K.E. et al. Synthesis of the (2S,4S)-stereoisomers of 4-(indol-1-yl) and 4-arylamino derivatives of 5-oxoproline, proline, and 2-hydroxymethylpyrrolidine. Russ Chem Bull 60, 873–881 (2011). https://doi.org/10.1007/s11172-011-0137-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-011-0137-4