Abstract
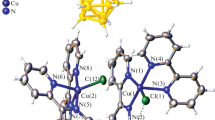
A series of the M(L)Cl2 · nH2O and {M(L)}2(OAc)4 complexes (M = NiII, CoII, and CuII; L is 3- and 4-(2-pyridyl)-1,3-benzothiazole) were synthesized by the reaction of L with MX2 · nH2O (X = Cl, OAc) in ethanol. The molecular and crystal structures of the CuL2(OAc)4 binuclear complex (L is 4-(2-pyridyl)benzothiazole) were determined by X-ray diffraction analysis. The copper atoms have a distorted tetragonal bipyramidal environment and are coordinated to the nitrogen atom of the pyridine moiety of the ligand and to two oxygen atoms of the bridging acetate ligands. The Cu-Cu distance is 2.6129(9) Å. The electrochemical behavior of the synthesized ligands and complexes was studied using the cyclic voltammetry and rotating disk electrode techniques in DMF solutions (0.1 M Bu4NClO4). The primary reduction of all the complexes under study is directed to the metal.
Similar content being viewed by others
References
D.-F. Shi, T. D. Bratshaw, S. Wrigley, C. J. McCall, P. Lelieveld, I. Fichtner, M. F. G. Stevens, J. Med. Chem., 1996, 39, 3375.
T. D. Bratshaw, S. Wrigley, D.-F. Shi, R. J. Schultz, K. D. Paull, M. F. G. Stevens, Br. J. Cancer, 1998, 77, 745.
M. Lezkano, W. Al-Soufi, M. Novo, E. Rodriguez-Nunez, J. V. Tato, J. Agric. Food Chem., 2002, 50, 108.
Z.-P. Zuang, M.-P. Kung, A. Wilson, C.-W. Lee, K. Plossl, C. Hou, D. M. Holtzmann, H. F. Kung, J. Med. Chem., 2003, 46, 237.
G. Wells, J. M. Berry, T. D. Bratshaw, A. M. Burger, A. Seaton, B. Wang, A. D. Westwell, M. F. G. Stevens, J. Med. Chem., 2003, 46, 532.
D. J. Skalitzki, J. T. Marakovits, K. A. Maegley, A. Ekker, X.-H. Yu, Z. Hostomsky, S. E. Webber, B. W. Eastman, R. Almassy, J. Li, N. J. Curtin, D. R. Newell, A. H. Calvert, R. J. Griffin, B. T. Golding, J. Med. Chem., 2003, 46, 210.
R. J. Alaimo, S. S. Pelosi, C. J. Hatton, J. E. Gray, J. Med. Chem., 1974, 17, 775.
R. D. Haugwitz, R. G. Angel, G. A. Jacobs, B. V. Maurer, V. L. Narayanan, L. R. Cruthers, J. Szanto, J. Med. Chem., 1982, 25, 969.
R. D. Haugwitz, B. V. Maurer, G. A. Jacobs, V. L. Narayanan, L. R. Cruthers, J. Szanto, J. Med. Chem., 1979, 22, 1113.
J. A. Grim, H. G. Petring, Cancer Res., 1967, 27, 1278.
S. V. Kryatov, B. S. Mohanraj, V. V. Tarasov, O. P. Kryatova, E. V. Rybak-Akimova, B. Nithakki, J. F. Rusling, R. J. Staples, A. Y. Nazarenko, Inorg. Chem., 2002, 41, 923.
B. Xie, L. J. Wilson, D. M. Stanbury, Inorg. Chem., 2001, 40, 3606.
X.-F. He, C. M. Vogels, A. Decken, S. A. Westcott, Polyhedron, 2004, 23, 155.
N. Goswami, D. M. Eichhorn, Inorg. Chem., 1999, 38, 4329.
D. Zhu, Y. Xu, Y. Mei, Y. Sci, C. Tu, X. You, J. Mol. Struct., 2001, 559, 119.
L. Zhang, L. Liu, D. Jia, K. Yu, Struct. Chem., 2004, 15, 327.
M. Bakiler, I. V. Masliv, S. Akyuz, J. Mol. Struct., 1999, 476, 21.
F. A. Cotton, G. Wilkinson, Advanced Inorganic Chemistry, 2nd ed., J. Wiley and Sons, New York-London-Sydney, 1966.
E. K. Beloglazkina, I. V. Yudin, A. G. Mazhuga, A. A. Moiseeva, A. I. Tursina, N. V. Zyk, Izv. Akad. Nauk, Ser. Khim., 2006, 1738 [Russ. Chem. Bull., Int. Ed., 2006, 55, 1803].
K. P. Butin, A. A. Moiseeva, E. K. Beloglazkina, Yu. B. Chudinov, A. A. Chizhevskii, A. V. Mironov, B. N. Tarasevich, A. V. Lalov, N. V. Zyk, Izv. Akad. Nauk, Ser. Khim., 2005, 169 [Russ. Chem. Bull., Int. Ed., 2005, 54, 173].
E. K. Beloglazkina, A. A. Moiseeva, A. V. Churakov, I. S. Orlov, N. V. Zyk, G. A. K. Howards, K. P. Butin, Izv. Akad. Nauk, Ser. Khim., 2002, 436 [Russ. Chem. Bull., Int. Ed., 2002, 51, 467].
R. W. Hay, J. A. Crayston, T. J. Cromie, P. Lightfoot, D. C. L. de Alwis, Polyhedron, 1997, 16, 3557.
A. M. Bond, Modern Polarographic Methods in Analytical Chemistry, Marcell Dekker, New York, 1980.
E. K. Beloglazkina, S. Z. Vatsadze, A. G. Mazhuga, N. A. Frolova, R. B. Romashkina, N. V. Zyk, A. A. Moiseeva, K. P. Butin, Izv. Akad. Nauk, Ser. Khim., 2005, 2679 [Russ. Chem. Bull., Int. Ed., 2005, 54, 2771].
E. K. Beloglazkina, A. G. Mazhuga, I. V. Yudin, N. A. Frolova, N. V. Zyk, V. D. Dolzhikova, A. A. Moiseeva, R. D. Rakhimov, K. P. Butin, Izv. Akad. Nauk, Ser. Khim., 2006, 978 [Russ. Chem. Bull., Int. Ed., 2006, 55, 1015].
J. P. Collman, M. Marrokko, C. M. Elliott, M. L’her, J. Electroanal. Chem., 1981, 124, 113.
M. Thirumavalavan, P. Akilan, M. Kandaswamy, Inorg. Chem., 2003, 42, 3308.
E. K. Beloglazkina, A. G. Mazhuga, A. A. Moiseeva, M. G. Tsepkov, N. V. Zyk, Izv. Akad. Nauk, Ser. Khim., 2007, 339 [Russ. Chem. Bull., Int. Ed., 2007, 56, 351].
J. J. P. Stewart, J. Comput. Chem., 1989, 10, 209.
L. S. Liebeskind, J. Srogl, Org. Lett., 2002, 4, 979.
H. D. Porter, J. Am. Chem. Soc., 1954, 76, 127.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 565–571, March, 2008.
Rights and permissions
About this article
Cite this article
Beloglazkina, E.K., Yudin, I.V., Mazhuga, A.G. et al. Synthesis and electrochemical study of 3- and 4-(2-pyridyl)-1,3-benzothiazole complexes with transition metals (CoII, NiII, and CuII). Molecular structure of bis{(4-(2-pyridyl)-1,3-benzothiazole)copper(ii)} tetraacetate. Russ Chem Bull 57, 577–584 (2008). https://doi.org/10.1007/s11172-008-0090-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-008-0090-z