Abstract
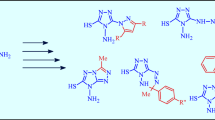
The reactions of hydrazide, mesyl hydrazide, succinyl hydrazide, and maleyl hydrazide of anthranilic acid with carbonyl compounds were studied, and new di-and tetrahydroquinazolin-4-one derivatives were prepared. The structure of one reaction product, viz., 3-methylsulfonylamino-2-(2-methylsulfonylaminophenyl)-1,2,3,4-tetrahydroquinazolin-4-one, was established by X-ray diffraction study of two crystal solvates of this compound. The characteristic features of the crystal packings of these solvates are discussed.
Similar content being viewed by others
References
S. Gabriel and J. Colman, Ber., 1904, 37, 3643.
C. Parkanyi, H. L. Yuan, B. H. E. Strömberg, and A. Evenzahav, J. Heterocycl. Chem., 1992, 29, 749.
P. K. Mohanta and K. Kim, Heterocycles, 2002, 57, 1471.
H. Böhme and H. Böing, Arch. Pharm., 1960, 293, 1011.
R. H. Klemm, J. Wang, and L. Hawkins, J. Heterocycl. Chem., 1995, 32, 1039.
R. H. Klemm, T. J. R. Weakley, R. D. Gilbertson, and Yang-Heon-Song, J. Heterocycl. Chem., 1998, 35, 1269.
J. Lessel, Arch. Pharm. (Weinheim), 1994, 327, 571.
L. A. Shemchuk, Zh. Org. Khim., 1998, 34, 568 [Russ. J. Org. Chem., 1998, 34 (Engl. Transl.)].
L. Yu. Ukhin and L. G. Kuz’mina, Izv. Akad. Nauk, Ser. Khim., 2004, 2165 [Russ. Chem. Bull., Int. Ed., 2004, 53, 2262].
D. Meth-Cohn, B. Narine, and B. Tarnowski, Tetrahedron Lett., 1997, 33, 3111.
L. Yu. Ukhin, K. Yu. Suponitskii, and V. G. Kartsev, Khim. Prirod. Soedin., 2003, 395 [Chem. Nat. Compd., 2003 (Engl. Transl.)].
SAINT. Version 6.02A, Bruker AXS Inc., Madison (Wisconsin, USA), 2001.
SHELXTL-Plus, Release 5.10, Bruker AXS Inc., Madison (Wisconsin, USA), 1997.
Author information
Authors and Affiliations
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 7, pp. 1183–1191, July, 2006.
Rights and permissions
About this article
Cite this article
Ukhin, L.Y., Kuz’mina, L.G. Synthesis of new 3,4-di-and 1,2,3,4-tetrahydroquinazolin-4-one derivatives and X-ray diffraction study of crystal solvates of 3-methylsulfonylamino-2-(2-methylsulfonylaminophenyl)-1,2,3,4-tetrahydroquinazolin-4-one. Russ Chem Bull 55, 1229–1238 (2006). https://doi.org/10.1007/s11172-006-0404-y
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0404-y