Abstract
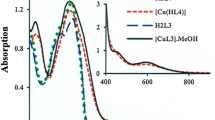
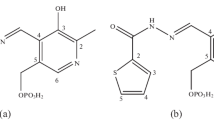
A series of CuII, NiII, and CoII complexes with 5-(pyridylmethylidene)-substituted 2-thiohydantoins (L) were synthesized by the reactions of the corresponding organic ligands with MCl2·nH2O. The resulting complexes have the composition LMCl2 (M = Cu or Ni) or L2MCl2 (M = Co). The reactions with N(3)-unsubstituted thiohydantoins afford complexes containing four-membered metallacycles, in which the metal ion is coordinated by the S and N(3) atoms of the thiohydantoin ligand. The reactions of N(3)-substituted thiohydantoins give complexes in which the S and N(1) atoms are involved in coordination. Study by IR spectroscopy demonstrated that the pyridine nitrogen atom is not involved in coordination. Based on the results of electrochemical study of the ligands and complexes by cyclic voltammetry and calculation of their frontier orbitals by the PM3(tm) method, the mechanism of oxidation and reduction of these compounds was proposed. In the first reduction and oxidation steps, the metal atom in the copper and nickel complexes remains, apparently, intact, and these processes occur with the involvement of the ligand fragments, viz., the coordinated thiohydantoin ligand and chloride anion, respectively. In the cobalt complexes, the first reduction step occurs at the ligand; the first oxidation state, at the metal atom. Measurements of the contact angle of aqueous wetting and electrochemical study demonstrated that carboxy-containing 2-thiohydantoins and their complexes can be adsorbed on the cystamine-modified gold surface. The structures of the complexes on the surface differ from the structures of these complexes in solution.
Similar content being viewed by others
References
V. Chazeau, M. Cossac, and A. Boucherle, Eur. J. Med. Chem., 1992, 27, 615.
K. Kiec-Kononowich and J. Karolak-Wojciechowska, Phosphorus, Sulfur, Silicon, 1992, 73, 235.
A. El-Barbary, Y. Aly, A. Hashem, and A. El-Shehawy, Phosphorus, Sulfur, Silicon, 2000, 160, 77.
D. Kushev, G. Gorneva, V. Enchev, E. Naydenova, J. Popova, S. Taxirov, L. Maneva, K. Grancharov, and N. Spassovska, J. Inorg. Biochem., 2002, 89, 203.
J. A. Grim and H. G. Petring, Cancer Res., 1967, 27, 1278.
J. S. Casas, E. E. Castellano, A. Macfas, N. Playa, A. Sanchez, J. Sordo, J. M. Varela, and E. Vasques-Lopez, Polyhedron, 2001, 20, 1845.
M. Arca, F. Demartin, F. Davillanova, A. Garau, F. Isaia, V. Lippolis, and G. Verani, Inorg. Chem., 1998, 37, 4164.
A. M. A. Hassaan, Sulfur Lett., 1991, 13, 1.
R. S. Srivastava, R. R. Srivastava, and H. N. Bhargava, Bull. Soc. Chim. Fr., 1991, 128, 671.
M. M. Chowdhry, D. M. Mingos, A. J. White, and D. J. Williams, J. Chem. Soc., Perkin Trans. 1, 2001, 20, 3495.
M. Kumar, A. Shaudhary, and T. Sharma, Ind. J. Chem., Sect. A, 1986, 25, 281.
J. Casas, E. Castellano, A. Macfas, N. Playa, A. Sanchez, J. Sordo, and J. Zukerman-Schpector, Inorg. Chim. Acta, 1995, 238, 129.
M. M. Chowdhry, A. Burrows, D. M. Mingos, A. J. White, and D. J. Williams, J. Chem. Soc., Chem. Commun., 1995, 1521.
J. S. Casas, E. E. Castellano, M. D. Couce, N. Playa, A. Sanchez, J. Sordo, J. M. Varela, and J. Zukerman-Schpector, J. Coord. Chem., 1999, 47, 299.
J. Casas, A. Castineiras, N. Playa, A. Sanchez, J. Sordo, J. Varela, and E. Vazquez-Lopez, Polyhedron, 1999, 18, 3653.
A. G. Majouga, E. K. Beloglazkina, S. Z. Vatsadze, A. A. Moiseeva, F. S. Moiseev, K. P. Butin, and N. V. Zyk, Mendeleev Commun., 2005, 48.
M. M. Chowdhry, D. M. Mingos, A. J. White, and D. J. Williams, J. Chem. Soc., Chem. Commun., 1996, 899.
A. G. Majouga, E. K. Beloglazkina, S. Z. Vatsadze, N. A. Frolova, and N. V. Zyk, Izv. Akad. Nauk, Ser. Khim., 2004, 2734 [Russ. Chem. Bull., Int. Ed., 2004, 53, 2850].
S.-F. Tan, K.-P. Ang, and Y.-F. Fong, J. Chem. Soc., Perkin Trans. 2, 1986, 1941.
E. K. Beloglazkina, S. Z. Vatsadze, A. G. Majouga, N. A. Frolova, R. B. Romashkina, N. V. Zyk, A. A. Moiseeva, and K. P. Butin, Izv. Akad. Nauk, Ser. Khim., 2005, 2679 [Russ. Chem. Bull., Int. Ed., 2005, 54, 2771].
A. G. Majouga, Ph. D. (Chem.) Thesis, M. V. Lomonosov Moscow State University, Moscow, 2005, 131 pp. (in Russian).
N. Goswami and D. M. Eichhorn, Inorg. Chem., 1999, 38, 4329.
D. Zhu, Y. Xu, Y. Mei, Y. Sci, C. Tu, and X. You, J. Mol. Struct., 2001, 559, 119.
L. Zhang, L. Liu, D. Jia, and K. Yu, Struct. Chem., 2004, 15, 327.
M. Bakiler, I. V. Masliv, and S. Akyuz, J. Mol. Struct., 1999, 476, 21.
I. Kuzniarska-Biernacka, A. Bartecki, and K. Kurzak, Polyhedron, 2003, 22, 997.
F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, 2 ed., J. Wiley and Sons, New York-London-Sydney, 1966.
S. Darwish, H. M. Fahmy, M. A. Abdel Aziz, and A. A. El Maghraby, J. Chem. Soc., Perkin Trans. 2, 1981, 344.
H. M. Fahmy, M. A. Abdel Aziz, and A. H. Badran, J. Electroanal. Chem., 1981, 127, 103.
M. A. Aboutabl, H. M. Fahmy, M. A. Abdel Aziz, and H. Abdel Rahman, J. Chem. Techn. Biotechn., 1983, 33A, 286.
G. M. Abou-Elenien, N. A. Ismail, and A. A. Magd Eldin, Monatsh. Chem., 1992, 123, 1117.
J. J. P. Stewart, J. Comput. Chem., 1989, 10, 209.
K. V. Gobi, K. Tokuda, and T. Ohsaka, J. Electroanal. Chem., 1998, 444, 145.
W. Yang, E. Chow, G. Willett, D. B. Hibbert, and J. J. Gooding, Analyst, 2003, 128, 712.
H. Y. Hu, A. M. Yu, and H. Y. Chen, J. Electroanal. Chem., 2001, 516, 119.
H. Z. Yu, J. W. Zhao, Y. Q. Wang, S. M. Cai, and Z. F. Liu, J. Electroanal. Chem., 1997, 438, 221.
H. Finklea and D. Hashew, J. Am. Chem. Soc., 1992, 114, 3173.
T. Johnson and B. Nicolet, J. Am. Chem. Soc., 1911, 33, 1973.
Author information
Authors and Affiliations
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 978–990, June, 2006.
Rights and permissions
About this article
Cite this article
Beloglazkina, E.K., Majouga, A.G., Yudin, I.V. et al. 5-(Pyridylmethylidene)-substituted 2-thiohydantoins and their complexes with CuII, NiII, and CoII: Synthesis, electrochemical study, and adsorption on the cystamine-modified gold surface. Russ Chem Bull 55, 1015–1027 (2006). https://doi.org/10.1007/s11172-006-0371-3
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0371-3