Abstract
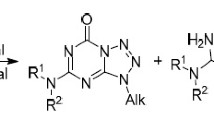
Quantum chemical methods involving studies of transition states of the reaction showed that the main products of N-alkylation of prototropic 2,3-dihydroimidazo[2,1-b]quinazolin-1(10)H-5-one (1) in the gas phase and under neutral conditions in solution occurring via the SN2 mechanism should be N(10)-alkyl-substituted derivatives formed from the 1H-tautomer. Minor N(1)-substituted derivatives in solution can be produced from both tautomers. For the alkylation of the free N-anion of compound 1, position 1 is attacked first. Validity of conclusions concerning the overall regioselectivity of the reaction was confirmed experimentally. In the absence of solvent, the alkylation proceeds abnormally with a sharp increase in the content of the 1-substituted isomers up to inversion of the regioselectivity of the reaction, which is explained by the participation in the process of the H-bonded dimer of the substrate (1a)2, which undergoes alkylation via the cryptoanionic mechanism.
Similar content being viewed by others
References
G. H. Hardtmann, G. Koletar, and O. R. Pfister, J. Med. Chem., 1975, 18, 447.
G. H. Hardtmann, US Pat. 3833652; http://www.chemweb.com/databases/patents/.
G. H. Hardtmann, US Pat. 3912731; http://www.chemweb.com/databases/patents/.
G. H. Hardtmann, US Pat. 3919210; http://www.chemweb.com/databases/patents/.
G. H. Hardtmann, US Pat. 3969506; http://www.chemweb.com/databases/patents/.
N. P. Peet and Sh. Sunder, US Pat. 4871732; http://www.chemweb.com/databases/patents/.
G. H. Hardtmann, US Pat. 3598823; http://www.chemweb.com/databases/patents/
T. Jen, B. Dienel, H. Bowman, J. Petta, A. Helt, and B. Loev, J. Med. Chem., 1972, 15, 727.
E. Takeuchi and T. Sato, Jpn Pat. 61115083; http://v3.espacenet.com.
M. O. Sinnokrot and C. D. Sherrill, J. Am. Chem. Soc., 2004, 126, 7690.
H.-S. Lee, D.-Y. Cheong, S.-D. Yoh, W.-S. Kim, Y.-W. Kwak, Y.-T. Park, and J.-K. Lee, Bull. Korean Chem. Soc., 2001, 22, 633.
G. S. Hammond, J. Am. Chem. Soc., 1955, 77, 334.
T. N. Truong, T.-T. T. Truong, and E. V. Stefanovich, J. Chem. Phys., 1997, 107, 1881.
J. Gao and X. Xia, J. Am. Chem. Soc., 1993, 115, 9667.
S. P. Webb and M. S. Gordon, J. Phys. Chem. A, 1999, 103, 1265.
M. Sola, A. Lledos, M. Duran, J. Bertran, and J. M. Abboud, J. Am. Chem. Soc., 1991, 113, 2873.
J. March, Advaced Organic Chemistry, Reactions, Mechanisms and Structure, Wiley-Interscience Publication, New York, 1985.
D. K. Bohme and A. B. Raksit, J. Am. Chem. Soc., 1984, 106, 3447.
E. Humeres, R. J. Nunes, V. G. Machado, M. D. G. Gasques, and C. Machado, J. Org. Chem., 2001, 66, 1163.
K. C. Westaway, Y. Gao, and Y. Fang, J. Org. Chem., 2003, 68, 3084.
M. L. Glowka, A. Olczak, and L. Korzycka, J. Chem. Crystallogr., 1994, 24, 725.
W. J. Le Noble, Synthesis, 1970, 1.
K. Hori, J.-L. M. Abboud, C. Lim, M. Fujio, and Y. Tsuno, J. Org. Chem., 1998, 63, 4228.
S. D. Yoh, M.-K. Lee, K.-J. Son, D.-Y. Cheong, I. Han, and K.-T. Shim, Bull. Korean Chem. Soc., 1999, 20, 466.
Y. Takata, Y. Huang, J. Komoto, T. Yamada, K. Konishi, H. Ogawa, T. Gomi, M. Fujioka, and F. Takusagawa, Biochemistry, 2003, 42, 8394.
J. Komoto, T. Yamada, Y. Takata, K. Konishi, H. Ogawa, T. Gomi, M. Fujioka, and F. Takusagawa, Biochemistry, 2004, 43, 14385.
P. Velichkova and F. Himo, J. Phys. Chem. B, 2005, 109, 8216.
P. Velichkova and F. Himo, J. Phys. Chem. B, 2005, 109; ASAP Web Release, Date: 07-Dec-2005.
W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis, and J. A. Montgomery, J. Comput. Chem., 1993, 14, 1347.
M. N. Glukhovstev, A. Pross, M. P. McGrath, and L. Radom, J. Chem. Phys., 1995, 103, 1878.
K. K. Irikura, R. D. Johnson III, and R. N. Kacker, J. Phys. Chem. A, 2005, 109, 8430.
Author information
Authors and Affiliations
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 876–887, May, 2006.
Rights and permissions
About this article
Cite this article
Morkovnik, A.S., Divaeva, L.N. & Kuz’menko, T.A. N-alkylation of 2,3-dihydroimidazo[2,1-b]quinazolin-1(10)H-5-one. On the cryptoanionic mechanism of N-substitution. Russ Chem Bull 55, 907–919 (2006). https://doi.org/10.1007/s11172-006-0351-7
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0351-7