Abstract
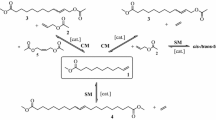
High (Z)-stereoselectivity of olefination of methyl α-phenylthioalkyl and methyl α-phenylthioalkenyl ketones with methyl trimethylsilylacetate decreases in the case of ketones containing a higher alkyl-substituent in place of the methyl group.
Similar content being viewed by others
References
N. Ya. Grigorieva, O. A. Pinsker, and A. M. Moiseenkov, Mendeleev Commun., 1994, 129.
N. Ya. Grigorieva, O. A. Pinsker, A. V. Buevich, and A. M. Moiseenkov, Izv. Akad. Nauk. Ser. Khim., 1995, 509 [Russ. Chem. Bull., 1995, 44, 492 (Engl. Transl.)].
N. Ya. Grigorieva, P. G. Tsiklauri, and O. A. Pinsker, Izv. Akad. Nauk. Ser. Khim., 1999, 1389 [Russ. Chem. Bull., 1999, 48, 1376 (Engl. Transl.)].
N. Ya. Grigorieva and P. G. Tsiklauri, Izv. Akad. Nauk. Ser. Khim., 2004, 635 [Russ. Chem. Bull., Int. Ed., 2004, 53, 665].
M. S. Brown and H. Rapoport, J. Org. Chem., 1963, 28, 3261.
Author information
Authors and Affiliations
Additional information
Dedicated to Corresponding Member of the Russian Academy of Sciences E. P. Serebryakov on the occasion of his 70th birthday.
__________
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 1020–1021, April, 2005.
Rights and permissions
About this article
Cite this article
Grigorieva, N.Y. Stereochemistry of ketone olefination with methyl trimethylsilylacetate. Russ Chem Bull 54, 1046–1047 (2005). https://doi.org/10.1007/s11172-005-0356-7
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-005-0356-7