Abstract
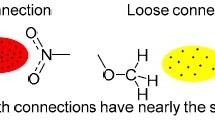
The nature and the energy of the intermolecular bifurcated N—H...N hydrogen bond in the crystal of 3-amino-6-(3,5-dimethylpyrazol-1-yl)-1,2,4,5-tetrazine were studied by analyzing the electron density distribution based on X-ray diffraction data. In contrast to two-center hydrogen bonds, the total energy of the N—H...N interaction is virtually independent of the geometric parameters of two contacts and is determined only by the nature of the interacting atoms.
Similar content being viewed by others
References
K. A. Lyssenko, I. L. Odinets, P. V. Kazakov, M. P. Pasechnik, and M. Yu. Antipin, Izv. Akad. Nauk, Ser. Khim., 2005, 553 [Russ. Chem. Bull., Int. Ed., 2005, 54, 560].
G. A. Jeffrey and W. Saenger, Hydrogen Bonding in Biological Structures, Springer-Verlag, Berlin, 1991.
A. V. Iogansen, Spectrochimica Acta, 1999, A55, 1585
L. M. Epstein and E. S. Shubina, Coord. Chem. Rev., 2002, 231, 165.
T. Steiner, Angew. Chem., Int. Ed., 2002, 41, 58.
R. F. W. Bader, Atoms in Molecules. A Quantum Theory, Clarendron Press, Oxford, 1990.
E. Espinosa, E. Mollins, and C. Lecomte, Chem. Phys. Lett., 1998, 285, 170.
I. Rozas, I. Alkorta, and J. Elguero, J. Phys. Chem. A, 1998, 102, 9925.
R. S. Gopalan, G. U. Kulkarni, E. Subramanian, and S. Renganayaki, J. Mol. Struct., 2000, 524, 169.
J. Ellena, A. E. Goeta, J. A. K. Howard, and G. Punte, J. Phys. Chem. A, 2001, 105, 8696.
D. E. Chavez, M. A. Hiskey, and R. D. Gilardi, Angew. Chem., Int. Ed., 2000, 39, 1791.
C. Glidewell, P. Lightfoot, B. J. L. Royles, and D. M. Smith, J. Chem. Soc., Perkin Trans. 2, 1997, 1167.
A. Klein, E. J. L. McInnes, T. Scheiring, and S. Zalis, J. Chem. Soc., Faraday Trans., 1998, 94, 2979.
Cambridge Crystallographic Database, release 2003.
M. Yu. Antipin, T. V. Timofeeva, D. S. Yufit, and J. Sauer, Izv. Akad. Nauk, Ser. Khim., 1995, 2443 [Russ. Chem. Bull., 1995, 44, 2337 (Engl. Transl.)].
K. A. Lyssenko, M. Yu. Antipin, and V. N. Khrustalev, Izv. Akad. Nauk, Ser. Khim., 2001, 1465 [Russ. Chem. Bull., Int. Ed., 2001, 50, 1539].
J. Bernstein, Polymorphism in Molecular Crystals, Clarendon Press, Oxford, 2002.
M. D. Coburn, G. A. Buntain, B. W. Harris, M. A. Hiskey, K.-Y. Lee, and D. G. Ott, J. Heterocyclic Chem., 1991, 28, 2049.
SMART. Bruker Molecular Analysis Research Tool, v. 5.059, 1998, Bruker AXS, Madison, Wisconsin, USA.
G. M. Sheldrick, SADABS, Bruker AXS Inc., Madison, WI 53719, USA, 1997.
G. M. Sheldrick, SHELXTL-97, Version 5.10, Bruker AXS Inc., Madison, WI 53719, USA, 1998.
N. K. Hansen and P. Coppens, Acta Crystallogr., 1978, A34, 909.
T. Koritsanszky, S. T. Howard, T. Richter, P. Macchi, A. Volkov, C. Gatti, P. R. Mallinson, L. J. Farrugia, Z. Su, and N. K. Hansen, XD — A Computer Program Package for Multipole Refinement and Topological Analysis of Charge Densities from Diffraction Data, 2003.
F. L. Hirshfeld, Acta Crystallogr., 1976, A32, 239.
D. A. Kirzhnitts, Zh. Eksp. Teor. Fiz., 1957, 5, 64 [J. Exp. Theor. Phys. USSR, 1957, 5, 64 (Engl. Transl.)].
A. Stash and V. Tsirelson, WinXPRO — a Program for Calculation of the Crystal and Molecular Properties Using the Model Electron Density, Moscow (Russia), 2001. A further information available at http://xray.nifhi.ru/wxp/
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, and J. A. Pople, Gaussian 98, Revision A.9, Gaussian, Inc., Pittsburgh PA, 1998.
D. N. Laikov, Chem. Phys. Lett., 1997, 281, 151.
MORPHY98, A Topological Analysis Program Written by P. L. A. Popelier with a Contribution from R. G. A. Bone (UMIST, Engl, EU); (b) P. Popelier, Chem. Phys. Lett., 1994, 228, 160.
Author information
Authors and Affiliations
Additional information
Dedicated to Academician V. I. Minkin on the occasion of his 70th birthday.
__________
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 903–910, April, 2005.
Rights and permissions
About this article
Cite this article
Lyssenko, K.A., Lyubetsky, D.V., Sheremetev, A.B. et al. Nature of weak inter- and intramolecular interactions in crystals 4. Bifurcated N—H...N bond in a crystal of 3-amino-6-(3,5-dimethylpyrazol-1-yl)-1,2,4,5-tetrazine. Russ Chem Bull 54, 924–932 (2005). https://doi.org/10.1007/s11172-005-0336-y
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-005-0336-y