Abstract
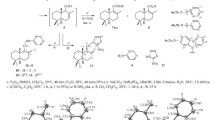
A convenient synthesis of drimenol by treatment of readily available drimane-8α, 11-diol 11-monoacetate with sulfuric acid in ethanol under mild conditions was developed. Oxidation of drimenol with selenium dioxide gives known drim-7-ene-9α, 11-diol and drim-7-ene-11,12-diol as the major products.
Similar content being viewed by others
References
B. J. M. Jansen and A. de Groot, Nat. Prod. Rep., 1991, 8, 319.
J. Lopez, J. Sierra, and M. Cortes, Chem. Lett., 1986, 2073.
M. Cortes, I. Razmilic, and J. Lopez, J. Nat. Prod., 1990, 53, 1369.
A. F. Barrero, M. Cortes, E. A. Manzaneda, E. Cabrera, R. Chahboun, M. Lara, and A. R. Rivas, J. Nat. Prod., 1999, 62, 1488.
B. J. M. Jansen and A. de Groot, Nat. Prod. Rep., 1991, 8, 309.
M. Toyota, Y. Ooiso, T. Kusuyama, and Y. Asakawa, Phytochemistry, 1994, 35, 1263.
B. P. Ying, G. Paiser, I. I. Ji, K. Mathias, D. Tutko, and Y. S. Hwang, Phytochemistry, 1995, 38, 909.
A. F. Barrero, E. A. Manzaneda, J. Altarejos, S. Salido, J. M. Ramos, M. S. J. Simmonds, and W. M. Blaney, Tetrahedron, 1995, 51, 7435.
B. M. F. Lagnel, Ch. Morin, and A. de Groot, Synthesis, 2000, 1907.
K. I. Kuchkova, Y. M. Chumakov, Y. A. Simonov, G. Bocelli, A. A. Panasenko, and P. F. Vlad, Synthesis, 1997, 1045.
P. F. Vlad, M. N. Koltsa, and G. N. Mironov, Izv. Akad. Nauk, Ser. Khim., 1997, 896 [Russ. Chem. Bull., 1997, 46, 855 (Engl. Transl.)].
S. Poigny, T. Huor, M. Guyot, and M. Samadi, J. Org. Chem., 1999, 64, 9318.
P. F. Vlad, G. V. Kryshtal’, and G. V. Lazur’evskii, Zh. Obshch. Khim., 1967, 37, 2187 [J. Gen. Chem. USSR, 1967, 37 (Engl. Transl.)].
I. Razmilic, J. Lopez, and M. Cortes, Synth. Commun., 1993, 23, 1155.
K. Mori and H. Watanabe, Tetrahedron, 1986, 42, 273.
G. D. Brown, Phytochemistry, 1994, 35, 975.
J. G. Urones, J. S. Marcos, B. G. Perez, D. Diez, A. M. Lithgow, P. M. Gomes, P. Basabe, and N. M. Garrido, Tetrahedron, 1994, 50, 10995.
M. L. Oyarzun, M. Cortes, and J. Sierra, Synth. Commun., 1982, 12, 951.
I. Razmilic, J. Lopez, J. Sierra, and M. Cortes, Synth. Commun., 1987, 17, 95.
D. M. Hollinshead, S. C. Howell, S. V. Ley, M. Mahon, N. M. Ratcliffe, and P. A. Worthington, J. Chem. Soc., Perkin Trans. 1, 1983, 1579.
H. H. Appel, C. J. W. Brooks, and K. H. Overton, J. Chem. Soc., 1959, 3322.
Author information
Authors and Affiliations
Additional information
__________
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 12, pp. 2745–2748, December, 2004.
Rights and permissions
About this article
Cite this article
Kuchkova, K.I., Aricu, A.N., Dragalin, I.P. et al. Convenient synthesis of drimenol and its oxidation with selenium dioxide. Russ Chem Bull 53, 2862–2865 (2004). https://doi.org/10.1007/s11172-005-0203-x
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-005-0203-x