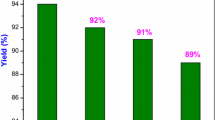
Abstract
In spite of having significant pharmacological importance, scalable synthesis of 2,2,4-trimethyl-1,2-H-dihydroquinoline (TMQ) is always being cumbersome due to harmful solvents, drastic reaction conditions, and high recovery cost of homogeneous catalysts. Heterogeneous catalytic condensation of aniline with acetone was employed here to synthesize TMQ. As efficient materials, Zn2+-, Sn2+-, and Cu2+-exchanged tungstophosphoric acid (TPA) supported on γ-Al2O3 was synthesized by microwave-assisted hydrothermal method. The synthesized catalysts were thoroughly characterized by XRD, Raman, FE-SEM, etc. and were screened for condensation reaction. Among the metal-exchanged catalysts, Zn0.5TPA/Al2O3 showed highest aniline conversion with the highest yield of TMQ up to three consecutive cycles. The acidic sites over the catalysts were probed by pyridine-adsorbed FT-IR spectra and NH3-TPD studies. The reaction conditions were optimized, and plausible reaction mechanistic pathway was derived from FT-IR and GC–MS data.










Similar content being viewed by others
References
A.R. Katritzky, C.W. Rees, E.F.V. Scriven, Comprehensive Heterocyclic Chemistry, II edn. (Elsevier, Oxford, 1996)
J.V. Johnson, B.S. Rauckman, D.P. Baccanari, B. Roth, J. Med. Chem. 32, 1942 (1989)
T. Aono, T. Doi K. Fukatsu, JP 042823701992 A2 (1992)
G. de Nanteuil, J. Duhault, D. Ravel, Y. Herve, EP 528734 (1993)
B.C. Pearce, J.J. Wright, US Patent 5411969 (1995)
T.K. Jones, M.E. Goldman, C.L.F. Pooley, D.T. Winn, J.E. Edwards, S.J. West, C.M. Tegley, L. Zhi, L.G. Hamann, WO 9619458 (1996)
M.J. Coughlan, S.W. Elmore, M.E. Kort, P.R. Kym, J.L. Moore, J.K. Pratt, A.X. Wang, J.P. Edwards, T.K. Jones, WO 9941256 (1999)
E.M. Bickhoff, A.L. Livingston, J. Guggolz, C.R. Thompson, A. Carotene, J. Agric. Food. Chem. 24, 1229 (1954)
W. Helmut, S. Worth, Leslie, US Patent 5147844 A (1992)
A.R. Katritzky, S. Rachwal, B. Rachwal, Terrahedron 52, 15031 (1996)
Z.H. Skraup, Monatsh. Chem. 1, 316 (1880)
Z.H. Skraup, Monatsh. Chem. 2, 139 (1881)
Z.H. Skraup, Monatsh. Chem. 2, 587 (1881)
M.E. Theoclitou, L.A. Robinson, Tetrahedron Lett. 43, 3907 (2002)
J. Fotie, N. Massawe, B.T. Bhattarai, J.L. Rhodus, A.S. Thomas, D.S. Bohle, J. Org. Chem. 77, 2784 (2012)
R.U. Gutierrez, H.C. Correa, A.V. Jerezano, F. Delgado, J. Tamariz, J. Org. Chem. 78, 9614 (2013)
M. Kazuishi, H. Osamu, H. Yasumasa, Tetrahedron Lett. 44, 8925 (2003)
J.S. Yadav, K. Premalatha, M.S.R. Murty, J. Mol. Cat. A: Chem. 271, 161 (2007)
L.L. Genliang, Portscheller Helena, C. Malinakova, Organometallics 24, 945 (2005)
X.Y. Liu, P. Ding, J.S. Huang, C.M. Che, Org. Lett. 9, 2645 (2007)
C.S. Yi, S.Y. Yun, J. Am. Chem. Soc. 127, 17000 (2005)
Y. Luo, Z. Li, C. Li, J. Org. Lett. 7, 2675 (2005)
X.-Y. Hu, J.-C. Zhang, J.-X. Ji, Tetrahedron Lett. 52, 2903 (2011)
H. Waldmann, G.V. Karunakar, K. Kumar, Org. Lett. 10, 2159 (2008)
G. Li, H. Liu, Y. Wang, S. Zhang, S. Lai, L. Tang, J. Zhao, Z. Tanga, Chem. Commun. 52(11), 2304 (2016)
D. Kundu, S.K. Kundu, A. Majee, A. Hajra, J. Chin. Chem. Soc. 55, 1186 (2008)
M. Eda, T. Kuroda, S. Kaneko, Y. Aoki, M. Yamashita, C. Okumura, Y. Ikeda, T. Ohbora, M. Sakaue, N. Koyama, K. Aritomo, J. Med. Chem. 58(12), 4918 (2015)
Y. Li, W. Chunlei, J. Huang, S. Weike, Synth. Commun. 36, 3065 (2006)
M. Cheng, X. Yang, J. Li, C. Chen, J. Zhao, Yu. Wang, L. Sun, Chem. Eur. J. 18(50), 16196 (2012)
R. Chen, X. Yang, H. Tian, X. Wang, A. Hagfeldt, L. Sun, Chem. Mater. 19(16), 4007 (2007)
J. Fotie, M. Kaiser, D.A. Delfín, J. Manley, C.S. Reid, J.-M. Paris, T. Wenzler, L. Maes, K.V. Mahasenan, C. Li, K.A. Werbovetz, J. Med. Chem. 53(3), 966 (2010)
G. Parameswaram, S. Roy, RSC Adv. 8, 28461 (2018)
P. Ganji, S. Roy, Catal. Commun. 134, 105864 (2020)
J. Li, X. Wang, W. Zhu, F. Cao, ChemSusChem 2, 177 (2009)
S. Shraavan, S. Challagulla, S. Banerjee, S. Roy, Bull. Mater. Sci. 40(7), 1415 (2017)
P.K.R. Boppidi, P.M.P. Raj, S. Challagulla, S.R. Gollu, S. Roy, S. Banerjee, S. Kundu, J. Appl. Phys. 124(21), 214901 (2018)
S. Roy, B. Viswanath, M.S. Hegde, G. Madras, J. Phys. Chem. C 112(15), 6002 (2008)
S. Kamiguchi, I. Takahashi, H. Kurokawa, H. Miura, T. Chihara, Appl. Catal. A 309, 70 (2006)
A. Hegedu, Z. Hella, T. Vargadi, A. Potor, I. Gresitsb, Catal. Lett. 117, 99 (2001)
J. Nowickia, K. Jaroszewskab, E. Nowakowska-Bogdana, M. Szmatołaa, J. Llowskaa, Mol. Catal. 454, 94 (2018)
Ch. Ramesh Kumar, P.S. Sai Prasad, N. Lingaiah, J. Mol. Catal. A: Chem. 350, 83 (2011)
M.H. Haider, N.F. Dummer, D. Zhang, P. Miedziak, T.E. Davies, S.H. Taylor, D.J. Willock, D.W. Knight, D. Chadwick, G.J. Hutchings, J. Catal. 286, 206 (2012)
S.E. Denmark, S. Venkatraman, J. Org. Chem. 71, 1688 (2006)
J. Jang, J. Ha, B. Lim, Chem. Commun. 622, 1622 (2006)
Z. Chen, C.D. Pina, E. Falletta, M. Rossi, J. Catal. 227, 93 (2009)
G.D. Yadav, R.P. Kumbhar, S. Helder, Int. Rev. Chem. Eng. 4, 6 (2012)
A.K. Hajare, A.R. Jagdale, A.G. Gautham Shenoybb, N. Sinha, New J. Chem. 40, 4888 (2016)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krishna, B., Roy, S. Synthesis of 2,2,4-trimethyl-1,2-dihydroquinolines over metal-modified 12-tungstophosphoric acid-supported γ-Al2O3 catalyst. Res Chem Intermed 46, 4061–4077 (2020). https://doi.org/10.1007/s11164-020-04191-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04191-y