Abstract
A convenient, rapid, and highly efficient procedure for synthesis of 5-substituted-1H-tetrazoles was developed via multicomponent domino Knoevenagel condensation/1,3-dipolar cycloaddition reaction between aromatic aldehydes, malononitrile, and sodium azide in presence of Fe3O4 magnetic nanoparticles, using microwave irradiation and conventional heating, under solvent-free conditions. The procedure is efficient due to the low cost and nontoxicity of the catalyst, elimination of volatile and toxic solvents, very short reaction time, excellent product yield, easy methodology, and simple workup. The magnetite catalyst was recycled using an external magnet and could be reused in at least five consecutive runs, delivering high product yield.
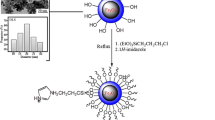
Graphical abstract
We illustrate that green, efficient, and recoverable Fe3O4 magnetic nanoparticles can catalyze Knoevenagel condensation/1,3-dipolar cycloaddition of aromatic aldehydes, malononitrile, and NaN3 to synthesize 5-substituted-1H-tetrazoles using microwave irradiation and conventional heating.






Similar content being viewed by others
References
M.J. Climent, A. Corma, S. Iborra, RSC Adv. 2, 1 (2012)
S.K. Das, S. Mondal, S. Chatterjee, A. Bhaumik, ChemCatChem 10, 11 (2018)
A. Ying, S. Liu, Y. Ni, F. Qiu, S. Xu, W. Tang, Catal. Sci. Technol. 4, 7 (2014)
M.R. Naimi-Jamal, S. Mashkouri, A. Sharifi, Mol. Divers. 14, 3 (2010)
P. Das, A. Dutta, A. Bhaumik, C. Mukhopadhyay, Green Chem. 16, 3 (2014)
H.R. Shaterian, K. Azizi, Res. Chem. Intermed. 41, 1 (2015)
S.K. Kundu, J. Mondal, A. Bhaumik, Dalton Trans. 42, 29 (2013)
R. Hiiisgm, Angew. Chem. 2, 11 (1963)
Z.H. Abood, R.T. Haiwal, S.M. Radhi, J. Babylon. Univ. Pure Appl. Sci. 21, 1 (2013)
S.K. Prajapti, A. Nagarsenkar, B.N. Babu, Tetrahedron Lett. 55, 24 (2014)
A. Kudelko, K. Jasiak, K. Ejsmont, Monatsh. Chem. 146, 2 (2015)
F. Abrishami, M. Ebrahimikia, F. Rafiee, Appl. Organometal. Chem. 29, 11 (2015)
J. Roh, K. Vávrová, A. Hrabálek, Eur. J. Org. Chem. 2012, 31 (2012)
V. Rama, K. Kanagaraj, K. Pitchumani, J. Org. Chem. 76, 21 (2011)
G. Aridoss, K.K. Laali, Eur. J. Org. Chem. 2011, 31 (2011)
J. Zabrocki, G.D. Smith, J.B. Dunbar, H. Iijima, G.R. Marshall, J. Am. Chem. Soc. 110, 17 (1988)
R. Romagnoli, P.G. Baraldi, M.K. Salvador, D. Preti, M. Aghazadeh Tabrizi, A. Brancale, R. Bortolozzi, J. Med. Chem. 55, 1 (2011)
J. Li, S.Y. Chen, J.J. Li, H. Wang, A.S. Hernandez, S. Tao, N. Flynn, J. Med. Chem. 50, 24 (2007)
A. Gagnon, S. Landry, R. Coulombe, A. Jakalian, I. Guse, B. Thavonekham, B. Simoneau, Bioorg. Med. Chem. Lett. 19, 4 (2009)
E. Vieira, J. Huwyler, S. Jolidon, F. Knoflach, V. Mutel, J. Wichmann, Bioorg. Med. Chem. Lett. 15, 20 (2005)
S. Kumar, S. Dubey, N. Saxena, S.K. Awasthi, Tetrahedron Lett. 55, 44 (2014)
P. Srihari, P. Dutta, R.S. Rao, J.S. Yadav, S. Chandrasekhar, P. Thombare, J. Mohapatra, A. Chatterjee, M.R. Jain, Bioorg. Med. Chem. Lett. 19, 19 (2009)
D.W. Nelson, R.J. Gregg, M.E. Kort, A. Perez-Medrano, E.A. Voight, Y. Wang, G. Grayson, M.T. Namovic, D.L. Donnelly-Roberts, W. Niforatos, P. Honore, M.F. Jarvis, C.R. Faltynek, W.A. Carroll, J. Med. Chem. 49, 12 (2006)
G. Ortar, A.S. Moriello, M.G. Cascio, L.D. Petrocellis, A. Ligresti, E. Morera, V.D. Marzo, Bioorg. Med. Chem. Lett. 18, 9 (2008)
A. Sarvary, A. Maleki, Mol. Divers. 19, 1 (2015)
A. Sarvary, A. Maleki, RSC Adv. 5, 75 (2015)
S.D. Guggilapu, S.K. Prajapti, A. Nagarsenkar, K.K. Gupta, B.N. Babu, Synlett 27, 8 (2016)
R.D. Padmaja, S. Rej, K. Chanda, Chin. J. Catal. 38, 11 (2017)
J. Roh, K. Vávrová, A. Hrabálek, Eur. J. Org. Chem. 2012, 31 (2012)
M. Parveen, F. Ahmad, A.M. Malla, A. Azaz, N. J. Chem. 39, 3 (2015)
A. Ghorbani-Choghamarani, Z. Moradi, G. Azadi, J. Sulfur Chem. 39, 3 (2018)
T. Tamoradi, B. Mehraban-Esfandiari, M. Ghadermazi, A. Ghorbani-Choghamarani, Res. Chem. Intermed. 44, 2 (2018)
M. Darabi, T. Tamoradi, M. Ghadermazi, A. Ghorbani-Choghamarani, Trans. Met. Chem. 42, 8 (2017)
F. Taghavi, M. Gholizadeh, A.S. Saljooghi, M. Ramezani, MedChemComm 8, 10 (2017)
Z.N. Tisseh, M. Dabiri, M. Nobahar, H.R. Khavasi, A. Bazgir, Tetrahedron 68, 6 (2012)
J. Safaei-Ghomi, S. Paymard-Samani, Chem. Heterocycl. Compd. 50, 11 (2015)
J. Safaei-Ghomi, S. Paymard-Samani, S. Zahedi, H. Shahbazi-Alavi, Z. Naturforsch. 70, 11 (2015)
S. Khaghaninejad, M.M. Heravi, T. Hosseinnejad, H.A. Oskooie, M. Bakavoli, Res. Chem. Intermed. 42, 3 (2016)
N. Ahmed, Z.N. Siddiqui, RSC Adv. 5, 22 (2015)
A. Banan, H. Valizadeh, A. Heydari, A. Moghimi, Appl. Organomet. Chem. 5, 22 (2017)
A.R. Faraji, S. Mosazadeh, F. Ashouri, J. Colloid Interface Sci. 15, 506 (2017)
Y. Rangraz, F. Nemati, A. Elhampour, J. Colloid Interface Sci. 509, 1 (2018)
H. Veisi, M. Pirhayati, A. Kakanejadifard, Tetrahedron Lett. 58, 45 (2017)
A. Baeza, G. Guillena, D.J. Ramón, ChemCatChem 8, 1 (2016)
N. Nami, D. Zareyee, M. Ghasemi, A. Asgharzadeh, M. Forouzani, S. Mirzad, S.M. Hashemi, J. Sulfur Chem. 38, 3 (2017)
M. Zarghani, B. Akhlaghinia, RSC Adv. 6, 45 (2016)
N.A. Aslam, S.A. Babu, D.K. Singh, A. Rana, Synlett 25, 15 (2014)
N. Koukabi, E. Kolvari, A. Khazaei, M.A. Zolfigol, B. Shirmardi-Shaghasemi, H.R. Khavasi, Chem. Commun. 47, 32 (2011)
A. Dastan, A. Kulkarni, B. Torok, Green Chem. 14, 1 (2012)
S.M. Joshi, R.B. Mane, K.R. Pulagam, V. Gomez-Vallejo, J. Llop, C. Rode, N. J. Chem. 41, 16 (2017)
M. Zhang, Y.H. Liu, Z.R. Shang, H.C. Hu, Z.H. Zhang, Catal. Commun. 88, 5 (2017)
S.L. Barbosa, M. Ottone, M.C. Santos, G.C. Junior, C.D. Lima, G.C. Glososki, N.P. Lopes, S.I. Klein, Catal. Commun. 68, 5 (2015)
N. Koukabi, E. Kolvari, M.A. Zolfigol, A. Khazaei, B.S. Shaghasemi, B. Fasahati, Adv. Synth. Catal. 354, 10 (2012)
E. Kolvari, N. Koukabi, M.M. Hosseini, M. Vahidian, E. Ghobadi, RSC Adv. 6, 9 (2016)
E. Kolvari, N. Koukabi, M.M. Hosseini, J. Mol. Catal. Chem. 397, 1 (2015)
S. Zolfagharinia, E. Kolvari, N. Koukabi, M.M. Hosseini, Arab. J. Chem. (2017)
B. Karami, S.J. Hoseini, K. Eskandari, A. Ghasemi, H. Nasrabadi, Sci. Technol. 2, 2 (2012)
X. Lu, H. Zhao, C. Feng, Q. Chen, Z. Zhang, C. Yang, X. Wang, RSC Adv. 7, 58 (2017)
M. Ma, Y. Zhang, W. Yu, H.Y. Shen, H.Q. Zhang, N. Gu, Colloid Surf. A Phys. Chem. Eng. Asp. 212, 2 (2003)
A.M. Ghasemzadeh, J. Safaei-Ghomi, S. Zahedi, J. Serb. Chem. Soc. 78, 6 (2013)
L. Jing, J. Wei, L. Zhou, Z. Huang, Z. Li, D. Wu, X. Zhou, Chem. Eur. J. 16, 36 (2010)
Acknowledgements
The authors gratefully acknowledge Semnan University Research Council for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akbarzadeh, P., Koukabi, N. & Kolvari, E. Three-component solvent-free synthesis of 5-substituted-1H-tetrazoles catalyzed by unmodified nanomagnetite with microwave irradiation or conventional heating. Res Chem Intermed 45, 1009–1024 (2019). https://doi.org/10.1007/s11164-018-3657-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3657-9