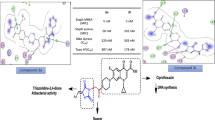
Abstract
We designed and synthesized a series of new ciprofloxacin–dithiocarbamate–benzyl hybrids 5a–n as potential antibacterial agents. All of the synthesized compounds were screened for in vitro antibacterial activity against four bacterial strains (Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa). The antibacterial results indicated that some of the synthesized compounds showed remarkable activity, comparable to their parent drug ciprofloxacin. Among the synthesized compounds, the 4-nitro derivative 5n, exhibited the most antibacterial activity against S. aureus, S. epidermidis, and E. coli. The antibacterial activity of this compound against S. aureus and E. coli was twofold higher than that of its parent ciprofloxacin. Molecular modeling of compound 5n was also investigated in the active site of E. coli DNA gyrase.




Similar content being viewed by others
References
I.M. Gould, Int. J. Antimicrob. Agents 32, S2 (2008)
D.M. Livermore, Int. J. Antimicrob. Agents 39, 283 (2012)
U. Theuretzbacher, Curr. Opin. Pharmacol. 11, 433 (2011)
W. Witte, C. Cuny, I. Klare, U. Nübel, B. Strommenger, G. Werner, Int. J. Med. Microbiol. 298, 365 (2008)
K. Lal, P. Yadav, A. Kumar, A. Kumar, A.K. Paul, Bioorg. Chem. 77, 236 (2018)
C. Viegas-Junior, A. Danuello, V. da Silva Bolzani, E.J. Barreiro, C.A.M. Fraga, Curr. Med. Chem. 14, 1829 (2007)
F. Vafadarnejad, M. Mahdavi, E. Karimpour-Razkenari, N. Edraki, B. Sameem, M. Khanavi, M. Saeedi, T. Akbarzadeh, Bioorg. Chem. 77, 311 (2018)
M. Mohammadi-Khanaposhtani, M. Safavi, R. Sabourian, M. Mahdavi, M. Pordeli, M. Saeedi, S.K. Ardestani, A. Foroumadi, A. Shafiee, T. Akbarzadeh, Mol. Divers. 19, 787 (2015)
M. Mohammadi-Khanaposhtani, M. Shabani, M. Faizi, I. Aghaei, R. Jahani, Z. Sharafi, N.S. Zafarghandi, M. Mahdavi, T. Akbarzadeh, S. Emami, A. Shafiee, A. Foroumadi, Eur. J. Med. Chem. 112, 91 (2016)
M.L. Liu, H.Y. Guo, World Notes Antibiot. 27, 69 (2006)
H.J. Zeiler, K. Grohe, in Ciprofloxacin, ed. by H.C. Neu, D.S. Reeves (Vieweg + Teubner Verlag, Wiesbaden, 1986), p. 14
Y. Wang, G.L. Damu, J.S. Lv, R.X. Geng, D.C. Yang, C.H. Zhou, Bioorg. Med. Chem. Lett. 22, 5363 (2012)
G.E.D.A. Abuo-Rahma, H.A. Sarhan, G.F. Gad, Bioorg. Med. Chem. Lett. 17, 3879 (2009)
M.Y. Mentese, H. Bayrak, Y. Uygun, A. Mermer, S. Ulker, S.A. Karaoglu, N. Demirbas, Eur. J. Med. Chem. 67, 230 (2013)
T. Plech, B. Kaproń, A. Paneth, U. Kosikowska, A. Malm, A. Strzelczyk, P. Stączek, Ł. Świątek, B. Rajtar, M. Polz-Dacewicz, Eur. J. Med. Chem. 97, 94 (2015)
T. Plech, M. Wujec, U. Kosikowska, A. Malm, B. Rajtar, M. Polz-Dacewicz, Eur. J. Med. Chem. 60, 128–134 (2013)
Q. Li, J. Xing, H. Cheng, H. Wang, J. Wang, S. Wang, J. Zhou, H. Zhang, Chem. Biol. Drug Des. 85, 79 (2015)
P.C. Sharma, A. Jain, S. Jain, R. Pahwa, M.S. Yar, J. Enzyme Inhib. Med. Chem. 25, 577 (2010)
S. Wang, X.D. Jia, M.L. Liu, Y. Lu, H.Y. Guo, Bioorg. Med. Chem. Lett. 22, 5971 (2012)
N. Manav, A.K. Mishra, N.K. Kaushik, Spectrochim. Acta A 65, 32 (2006)
S.M. Mamba, A.K. Mishra, B.B. Mamba, P.B. Njobeh, M.F. Dutton, E. Fosso-Kankeu, Spectrochim. Acta A 77, 579 (2010)
F. Shaheen, A. Badshah, M. Gielen, M. Dusek, K. Fejfarova, D. de Vos, B. Mirza, Organomet. Chem. 692, 3019 (2007)
G. Cascio, L. Lorenzi, D. Caglio, E. Manghisi, F. Arcamone, G. Guanti, G. Satta, G. Morandotti, R. Sperning, Farmaco 51, 189 (1996)
E.J. Baron, S.M. Finegold, Diagn. Microbiol. 171 (1990)
Acknowledgements
This research was supported by grants from the research council of Tehran University of Medical Sciences and INSF (Iran National Science Foundation).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Esfahani, E.N., Mohammadi-Khanaposhtani, M., Rezaei, Z. et al. New ciprofloxacin–dithiocarbamate–benzyl hybrids: design, synthesis, antibacterial evaluation, and molecular modeling studies. Res Chem Intermed 45, 223–236 (2019). https://doi.org/10.1007/s11164-018-3598-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3598-3