Abstract
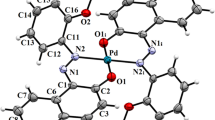
Palladium (II) complex with 4-tert-butylbenzoic hydrazide (TBBH)/triphenylphosphine (PPh3) ligands was successfully synthesized and characterized by X-ray, 1H-NMR, 13C-NMR, 31P-NMR, FT-IR, UV–Vis, elemental analysis and magnetic measurements. Palladium (II) complex possesses distorted square planar geometry bearing neutral ligands at trans positions and two chloride anions as counter ions. The homogenous catalytic activity of synthesized complex was investigated on Suzuki–Miyaura reaction between phenyboronic acid and selected aryl halides. The effect of solvent, temperature, base, reaction time and substrate type were investigated. From the results, water mixed solvents including methanol, ethanol, dimethyl formamide and dimethyl sulfoxide found to be the most suitable solvents rather than the pure ones. Potassium carbonate, as an inorganic base, found to be much more effective than organic base additives (triethylamine and trimethylamine). Increasing reaction temperature and time resulted to obtain higher product yields. The best biphenyl formation was observed in methanol/water (1:1) mixture with the conversion value of 98.5%, at 50 °C in 1-h reaction time.



Similar content being viewed by others
References
D. Cantillo, C.O. Kappe, Chem. Cat. Chem. 6, 3286 (2014)
M.H.P. Temprano, J.A. Casarez, P. Espinet, Chem. Eur. J. 18, 1864 (2012)
A. Talhami, L. Penn, N. Jaber, K. Hamza, J. Blum, Appl. Catal. A Gen. 312, 115 (2006)
S.K. Gurung, S. Thapa, A. Kafle, D.A. Dickie, R. Giri, Organ. Lett. 16, 1264 (2014)
A.-G. Choghamarani, A.A. Derakhshan, M. Hajjami, L. Rajabi, RSC Adv. 6, 94314 (2016)
F. Gonzales, G.C. Fu, JACS 128, 5360 (2006)
S. Ge, J.F. Hartwig, Angew. Chem. Int. Ed. 51, 12837 (2012)
K. Motoi, K. Kosuke, Y. Mitsuaki, H. Shuichi, I. Toshiyuki, Chem. Lett. 39, 1050 (2010)
W. Frank, T. Pautzsch, E. Klemm, Macromol. Chem. Phys. 202, 2535 (2001)
D.W. Old, J.P. Wolfe, S.L. Buchwald, J. Am. Chem. Soc. 120(37), 9722 (1998)
J.P. Wolfe, R.A. Singer, B.H. Yang, S.L. Buchwald, J. Am. Chem. Soc. 121, 9550 (1999)
B.P. Amit, H.C. Kishor, K. Premlata, Res. Chem. Intermed. 41, 2665 (2015)
F. Bellina, A. Carpita, R. Rossi, Synthesis 15, 2419 (2004)
S. Handa, Y. Wang, F. Gallou, B.H. Lipshutz, Science 349, 6252 (2015)
N.T.S. Phan, M.V.D. Sluys, C.W. Jones, Adv. Synth. Catal. 348, 609 (2006)
H.A. Dieck, R.F. Heck, J. Am. Chem. Soc. 96, 1133 (1974)
T. Sakamoto, E. Kato, Y. Negishi, H. Yamanaka, Chem. Pharm. Bull. 36, 1664 (1988)
L. Wang, A. Reis, A. Seifert, T. Philippi, S. Ernst, M. Jia, W.R. Thiel, Dalton Trans. 7, 3315 (2009)
Q.U. Ain, U. Ashiq, R.A. Jamal, M.M. Tahir, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 115, 683 (2013)
C. Xu, J.F. Gong, Y.H. Zhang, Y. Zhu, Y.-J. Wu, Aust. J. Chem. 60, 190 (2007)
Y.-C. Yang, P.H. Toy, Synlett 25, 1319 (2014)
A. Datta, K. Ebert, H. Plenio, Organometallics 22, 4685 (2003)
A. Bhumika, L. Samaresh, G. Anuradha Rakesh, D.P. Devendra, Inoranica Chimica Acta 471, 345 (2018)
D. Trisha, U. Hiroshi, N. Mahasweta, J. Solid State Chem. 260, 132 (2018)
SMART, Bruker AXS, (2000)
O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A.K. Howard, H. Puschmann, J. Appl. Cryst. 42, 339 (2009)
G.M. Sheldrick, Acta Cryst. A71, 3 (2015)
C.F. Macrae, P.R. Edgington, P. McCabe, E. Pidcock, G.P. Shields, R. Taylor, M. Towler, J. van de Streek, J. Appl. Crystallogr. 39, 453 (2006)
L. Lapo, G. Zufar, R. Andrea, Inorg. Chim. Acta 470, 100 (2018)
S. Maryam, H.K. Ali, Inorg. Chim. Acta 476, 20 (2018)
H. Joshua, G. Chris, S.G. Benjamin, R. Roland, Polyhedron 143, 138 (2018)
P. Pyali, J.B. Ray, B. Samaresh, Inorg. Chim. Acta 425, 67 (2015)
D. Jayita, G.R. Michael, B. Samaresh, Dalton Trans. 44, 13615 (2015)
D. Pitor, B. Anna, K. Mara, L. Tadeusz, Vib. Spectrosc. 40, 118 (2006)
P.I.S. Maia, A. Graminha, F.R. Pavan, C.Q.F. Leite, A.A. Batista, D.F. Back, E.S. Lag, J. Ellena, S.S. Lemos, H.S.S. Araujo, V.M. Deflon, J. Braz. Chem. Soc. 21, 1177 (2010)
L.M. Sousa, P.P. Corbi, A.L.B. Formiga, M. Lancelotti, I.M. Marzan, E.C. Pereira-Maia, G.V.W. Poelhsitz, J. Mol. Struct. 1097, 15 (2015)
M. Ghassemzadeh, S. Bahemmat, M. Mahmoodabadi, B.R. Rad, H.H. Monfared, E. Mottefakeri, B. Neumuller, Polyhedron 29, 3036 (2010)
M.M. Tamizh, B.F.T. Cooper, C.L.B. Mcdonald, R. Karvembu, Inorg. Chim. Acta 394, 391 (2013)
H. Unver, F. Yilmaz, J. Mol. Liq. 241, 875 (2017)
F.V. Rocha, C.V. Barra, S.S. Garrido, F.A. Manente, I.Z. Carlos, J. Ellena, A.S.C. Fuentes, A. Gautier, L. Morel, A.E. Mauro, A.V.G. Netto, J. Inorg. Biochem. 159, 165 (2016)
F.V. Rocha, C.V. Barra, A.E. Mauro, I. Karlos, Z. Nauton, M.E. Gozzi, A. Gautier, L. Morel, A.V.G. Netto, Eur. J. Inorg. Chem. 2013, 4499 (2013)
J. Xia, Y. Fu, G. He, X. Sun, X. Wang, Appl. Catal. B Environ. 200, 39 (2017)
T. Cuenca, M. Filice, J.M. Palomo, Enzyme Micro. Technol. 95, 242 (2016)
S. Keesara, M.R. Mandapati, S. Parvathaneni, Appl. Catal. A Gen. 496, 58 (2015)
Z. Beigi, A.H. Kianfar, G. Mohammednezhad, H. Görls, W. Plass, Polyhedron 134, 65 (2017)
T. Mahamo, M.M. Mogorosi, J.R. Moss, S.F. Maolie, J.C. Slootweg, K. Lammertsma, G.S. Smith, J. Organomet. Chem. 703, 34 (2012)
S.H. Priver, M.A. Bennett, A.C. Willis, S. Pottabathula, M.L. Kantam, S.K. Bargava, Dalton Trans. 43, 12000 (2014)
V. Pascanu, Q. Yao, A.B. Gomez, M. Gustafsson, Y. Yun, W. Wan, L. Samain, X. Zou, B.M. Matute, Chem. Eur. J. 19, 17483 (2013)
H. Yang, X. Han, Z. Ma, R. Wang, J. Liu, W. Ji, Green Chem. 12, 441 (2010)
Acknowledgements
The author thankfully acknowledges to the Anadolu University both Medicinal Plants and Medicine Research Centre for single crystal X-ray and department of chemistry for spectroscopic measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ünver, H. X-ray structure of palladium (II) complex and its catalytic activity on Suzuki–Miyaura reaction under mild conditions. Res Chem Intermed 44, 7835–7846 (2018). https://doi.org/10.1007/s11164-018-3589-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3589-4