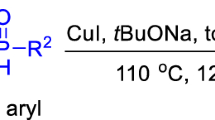Abstract
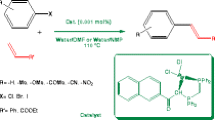
A novel and convenient approach to the synthesis of various tertiary phosphines via a copper-catalyzed cross-coupling of (hetero)aromatic bromide with secondary phosphines has been developed. The reaction employs cheap copper as the catalyst, 2,6-bis(N-methylaminomethyl)pyridine (L4) as a perfect ligand and KOtBu as a base; all reactions are carried out under argon atmosphere. A variety of sterically hindered and/or functionalized substrates were found to react under these reaction conditions to provide products in good to excellent yields. Moreover, ten new tertiary phosphines were first reported in this process.
Similar content being viewed by others
References
L.D. Quin, A Guide to Organophosphorus Chemistry (Wiely, New York, 2000)
A. Börner, Phosphorus Ligands in Asymmetric Catalysis–Synthesis and Application (Wiley-VCH, Weinheim, 2008)
H.A. McManus, P.J. Guiry, Chem. Rev. 4151, 104 (2004)
T. Baumgartner, R. Réau, Chem. Rev. 4681, 106 (2006)
M.N. Birkholz, Z. Freixa, W.N.M. van Leeuwen, Chem. Soc. Rev. 1099, 38 (2009)
W. Tang, X. Zhang, Chem. Rev. 3029, 103 (2003)
L. Ye, J. Zhou, Y. Tang, Chem. Soc. Rev. 1140, 37 (2008)
C.J. O’Brien, J.L. Tellez, Z.S. Nixon, L.J. Kang, A.L. Carter, S.R. Kunkel, K.C. Przeworski, G.A. Chass, Angew. Chem. Int. Ed. 6836, 48 (2009)
G.M. Kosolapoff, L. Maier, Organic Phosphorus Compounds (Wiley, New York, 1972)
Y. Zhou, Z.J. Gau, B. Su, J. Li, Z. Duan, F. Mathey, Org. Lett. 5722, 17 (2015)
P.W.N.M. van Leeuwen, P.C.J. Kamer, J.N.H. Reek, R. Dierkes, Chem. Rev. 2741, 100 (2000)
I.Y. Wan, J.E. McGrath, T. Kashiwagi, ACS Symp. Ser. 29, 599 (1995)
B. Buchner, L.B. Lockhart, J. Am. Chem. Soc. 755, 73 (1951)
R. Rabinowitz, J. Pellon, J. Org. Chem. 4623, 26 (1961)
M. Peer, J.C. de Jong, M. Kiefer, T. Langer, H. Rieck, H. Schell, P. Sennhenn, J. Sprinz, H. Steinhagen, B. Wiese, G. Helmchen, Tetrahedron 7547, 52 (1996)
E. Bélanger, M.F. Pouliot, J.F. Paquin, Org. Lett. 2201, 11 (2009)
D. Saha, R. Ghosh, A. Sarkar, Tetrahedron 3951, 69 (2013)
K.R. Seipel, Z.H. Platt, M. Nguyen, A.W. Holland, J. Org. Chem. 4291, 73 (2008)
M. Hayashi, T. Yamasaki, Y. Kobayashi, Y. Imai, Y. Watanabe, Eur. J. Org. Chem. 4956, 29 (2009)
I. Wauters, W. Debrouwer, C.V. Stevens, Beilstein J. Org. Chem. 1064, 10 (2014)
F. Alonso, Y. Moglie, G. Radivoy, M. Yus, Green Chem. 2699, 14 (2012)
Y. Moglie, M.J. González-Soria, I. Martín-García, G. Radivoy, F. Alonso, Green Chem. 4896, 18 (2016)
S.E. Tunney, J.K. Stille, J. Org. Chem. 748, 52 (1987)
S. Lunot, J. Thibonnet, A. Duchene, J.L. Parrain, Tetrahedron Lett. 8893, 41 (2000)
M. Al-Masum, T. Livinghouse, Tetrahedron Lett. 7731, 40 (1999)
R.K. Gujadhur, C.G. Bates, D. Venkataraman, Org. Lett. 4315, 3 (2001)
F.Y. Kwong, S.L. Buchwald, Org. Lett. 3517, 4 (2002)
A. Klapars, J.C. Antilla, X. Huang, S.L. Buchwald, J. Am. Chem. Soc. 7727, 123 (2001)
D. Ma, C. Xia, Org. Lett. 2583, 3 (2001)
R. Gujadhur, D. Venkataraman, Synth. Commun. 2865, 31 (2001)
R. Gujadhur, D. Venkataraman, J. Kintigh, Tetrahedron Lett. 4791, 42 (2001)
C. Bates, R. Gujadhur, D. Venkataraman, Org. Lett. 2803, 4 (2002)
C. Bates, P. Saejueng, J.M. Murphy, D. Venkataraman, Org. Lett. 4727, 3 (2002)
R. Gujadhur, D. Venkataraman, Tetrahedron Lett. 81, 44 (2003)
D. Van Allen, D. Venkataraman, J. Org. Chem. 4590, 68 (2003)
P. Saejueng, C.G. Bates, D. Venkataraman, Synthesis 1706, 10 (2005)
J.F. Marcoux, S. Doye, S.L. Buchwald, J. Am. Chem. Soc. 10539, 119 (1997)
M. Wolter, G. Nordmann, G.E. Job, S.L. Buchwald, Org. Lett. 973, 4 (2002)
A. Klapars, X.H. Huang, S.L. Buchwald, J. Am. Chem. Soc. 7421, 124 (2002)
F.Y. Kwong, A. Klapars, S.L. Buchwald, Org. Lett. 581, 4 (2002)
E.J. Hennessy, S.L. Buchwald, Org. Lett. 269, 4 (2002)
D. Gelman, L. Jiang, S.L. Buchwald, Org. Lett. 2315, 5 (2003)
L. Jiang, G.E. Job, A. Klapars, S.L. Buchwald, Org. Lett. 3667, 5 (2003)
A. Shafir, S.L. Buchwald, J. Am. Chem. Soc. 8742, 128 (2006)
N. Zheng, S.L. Buchwald, Org. Lett. 4749, 9 (2007)
G. Brasche, S.L. Buchwald, Angew. Chem. Int. Edit. 1932, 47 (2008)
G.O. Jones, P. Liu, K.N. Houk, S.L. Buchwald, J. Am. Chem. Soc. 6205, 132 (2010)
R. Zhu, S.L. Buchwald, Angew. Chem. Int. Edit. 12655, 52 (2013)
N. Niljianskl, S. Zhu, S.L. Buchwald, Angew. Chem. Int. Edit. 1638, 54 (2015)
M.W.J. Gribble, M.T. Pirnot, J.S. Bandar, R.Y. Liu, S.L. Buchwald, J. Am. Chem. Soc. 2192, 139 (2017)
V. Hornillos, M. Pérez, M.-M. Faňanás, B.L. Feringa, Chem. Eur. J. 5432, 19 (2013)
G.B. Hu, Y.X. Gao, Y.F. Zhao, Org. Lett. 4464, 16 (2014)
J. Ke, Y.L. Tang, H. Yi, Y.L. Li, Y.D. Cheng, C. Liu, A.W. Lei, Angew. Chem. Int. Edit. 6604, 54 (2015)
R. Beaud, R.J. Phipps, M.J. Gaunt, J. Am. Chem. Soc. 138, 40 (2016)
P. Ortiz, J.F. Collados, R.P. Jumde, E. Otten, S.R. Harutyunyan, Angew. Chem. Int. Edit. 3041, 56 (2017)
J.J. Becker, M.R. Gagne, Organometallics 4984, 22 (2003)
I.P. Beletskaya, A.V. Cheprakov, Coord. Chem. Rev. 2337, 248 (2004)
S. Thielges, P. Bisseret, J. Bisseret, Org. Lett. 681, 7 (2005)
C. Huang, X. Tang, H. Fu, Y. Jiang, Y. Zhao, J. Org. Chem. 5020, 71 (2006)
H. Rao, Y. Jin, H. Fu, Y. Jiang, Y. Zhao, Chem. Eur. J. 3636, 12 (2006)
Y. Gao, G. Wang, L. Chen, P. Chen, Y. Zhao, Y. Zhou, L.B. Han, J. Am. Chem. Soc. 7956, 131 (2009)
C.H. Lin, Y. Chi, M.W. Chung, Y.J. Chen, K.W. Wang, G.H. Lee, P.T. Chou, W.Y. Hung, H.C. Chiu, Dalton Trans. 1132, 40 (2011)
Y.H. Li, S. Das, S.L. Zhou, K. Junge, M. Beller, J. Am. Chem. Soc. 9727, 134 (2012)
B.Q. Xiong, M. Li, Y.X. Liu, Y.B. Zhou, C.Q. Zhao, M. Goto, S.F. Yin, L.B. Han, Adv. Synth. Catal. 781, 356 (2014)
H. Tinnermann, C. Wille, D.M. Alcarazo, Angew. Chem. Int. Edit. 8732, 53 (2014)
D.P. Zhao, T.M. Neubauer, B.L. Feringa, Nat. Commun. 6652, 6 (2015)
T. Ghosh, P. Maity, D. Kundu, B.C. Ranu, New J. Chem. 9556, 40 (2016)
P.B. Zhang, L.L. Zhang, Y.Z. Gao, G. Tang, Y.F. Zhao, RSC Adv. 60992, 6 (2016)
P.H.S. Paioti, K.A. Abboud, A. Aponick, ACS Catal. 2133, 7 (2017)
S. Kaye, J.M. Fox, F.A. Hicks, S.L. Buchwald, Adv. Synth. Catal. 789, 343 (2001)
D. Ma, Y. Zhang, J. Yao, S. Wu, F. Tao, J. Am. Chem. Soc. 12459, 120 (1998)
R.A. Altman, E.D. Koval, S.L. Buchwald, J. Org. Chem. 6190, 72 (2007)
R. Martin, S. Buchwald, Acc. Chem. Res. 1461, 41 (2008)
G. Evano, N. Blanchard, M. Toumi, Chem. Rev. 3054, 108 (2008)
F. Monnier, M. Taillefer, Angew. Chem. Int. Ed. 6954, 48 (2009)
M. Jahjah, M. Alame, S.P. Rostating, M. Lemaire, Tetrahedron Asymmetry 2305, 18 (2007)
H.R. Hays, J. Org. Chem. 3690, 33 (1968)
M.J.P. Harger, S. Westlake, Tetrahedron 1511, 38 (1982)
N.T. McDougal, J. Streuff, H. Mukherjee, S.C. Virgil, B.M. Stoltz, Tetrahedron Lett. 5550, 51 (2010)
A. Christiansen, C. Li, M. Garland, D. Selent, R. Ludwing, A. Spannenberg, W. Baumann, R. Franke, A. Börner, Eur. J. Org. Chem. 2733, 14 (2010)
Z.Y. Huang, Z. Liu, J.R. Zhou, J. Am. Chem. Soc. 15882, 133 (2011)
M. Sander, Chem. Ber. 93, 1220 (1960)
T.L. Emmick, R.L. Letsinger, J. Am. Chem. Soc. 3459, 90 (1968)
C.A. Busacca, J.C. Lorenz, N. Grinberg, N. Haddad, M. Hrapchak, B. Latli, H. Lee, P. Sabila, A. Saha, M. Sarvestani, S. Shen, R. Varsolona, X.D. Wei, C.H. Senanayake, Org. Lett. 4277, 7 (2005)
Z. Herseczki, I. Gergely, C. Hegedüs, Á. Szöllősy, J. Bakos, Tetrahedron Asymmetry 1673, 15 (2004)
H. Gulyás, J.B. Buchholz, E.C. Escudero-Adan, Z. Freixa, P.W. van Leeuwen, Chem. Eur. J. 3424, 13 (2007)
H.M. Walborsky, M. Topolski, J. Am. Chem. Soc. 3455, 114 (1992)
R.G. Yu, X.Y. Chen, S.F. Martin, Z.Q. Wang, Org. Lett. 1808, 19 (2017)
H. Lebel, V. Paquet, J. Am. Chem. Soc. 320, 126 (2004)
J. Li, H.W. Fu, P. Hu, Z.L. Zhang, X. Li, Y.X. Cheng, Chem. Eur. J. 13941, 18 (2012)
M. Murata, S.L. Buchwald, Tetrahedron 60, 7397 (2004)
Acknowledgements
The authors gratefully acknowledge the financial support from Hebei Chemical and Pharmaceutical College. The authors are thankful to Hebei University of Science and Technology for elemental, 13C NMR, 1H NMR, 19F NMR, and 31P NMR analysis.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, CJ., Lü, J., Zhang, ZX. et al. Copper-catalyzed C–P cross-coupling of secondary phosphines with (hetero)aromatic bromide. Res Chem Intermed 44, 4547–4562 (2018). https://doi.org/10.1007/s11164-018-3403-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3403-3