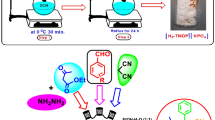
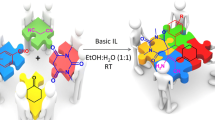
Abstract
In this research layered double hydroxides functionalized with (3r,5r,7r)-1-(3-(triethoxysilyl)propyl)-1,3,5,7-tetraazaadamantan-1-ium chloride (LDHs–TPTAACl) as a novel, green, efficient and recyclable heterogeneous ionic liquid were synthesized and characterized by various methods, such as FT-IR, TGA, SEM, EDX, TEM and XRD. The mentioned catalyst was applied as a new ionic liquid catalyst for the one-pot three-component synthesis of a new class of pyrido[2,3-d]pyrimidine derivatives through combination of aromatic aldehydes, 2,4-thiazolidinedione and N,N-dimethyl-6-amino uracil using water as solvent in high to excellent yield and short reaction times. The LDHs–TPTAACl catalyst could be simply recycled and reused up to five continuous runs with no substantial structural change and loss of activity.
Graphical Abstract














Similar content being viewed by others
References
T. Cheng, D. Zhang, H. Li, G. Liu, Green Chem. 16, 3401 (2014)
A. Nagaraju, B.J. Ramulu, G. Shukla, A. Srivastava, G.K. Verma, K. Raghuvanshi, M.S. Singh, Green Chem. 17, 950 (2015)
R.C. Cioc, E. Ruijter, R.V.A. Orru, Green Chem. 16, 2958 (2014)
P. Ravichandiran, B. Lai, Y. Gu, Chem. Rec. 17, 142 (2017)
V. Polshettiwar, R.S. Varma, Green Chem. 12, 743 (2010)
R. Liu, C. Dong, X. Liang, X. Wang, X. Hu, Chem, I. Ed. J. Org. Chem. 70, 729 (2005)
G. Stavber, M. Zupan, M. Jereb, S. Stavber, Org. Lett. 6, 4973 (2004)
C.P. Mehnert, Chem. A Eur. J. 11, 50 (2005)
A. Riisager, R. Fehrmann, S. Flicker, R. Van Hal, M. Haumann, P. Wasserscheid, Angew. Chemie Int. Ed. 44, 815 (2005)
M.V. Reddy, N.T.K. Lien, G.C.S. Reddy, K.T. Lim, Y.T. Jeong, Green Chem. 18, 4228 (2016)
J. He, M. Wei, B. Li, Y. Kang, D.G. Evans, X. Duan, Struct. Bond 89, 2006 (2006)
S. Yoon, J.-Y. Yun, J.-H. Lim, B. Yoo, J. Alloys Compd. 693, 964 (2017)
M. Shao, R. Zhang, Z. Li, M. Wei, D.G. Evans, X. Duan, Chem. Commun. 51, 15880 (2015)
H. Li, J. Li, C. Xu, P. Yang, D.H.L. Ng, P. Song, M. Zuo, J. Alloys Compd. 698, 852 (2017)
J. Wang, R. Zhu, B. Gao, B. Wu, K. Li, X. Sun, H. Liu, S. Wang, Biomaterials 35, 466 (2014)
N.T. Thao, N.D. Trung, D. Van Long, Catal. Lett. 146, 918 (2016)
E. Li, Z.P. Xu, V. Rudolph, Appl. Catal. B Environ. 88, 42 (2009)
W.Y. Hernandez, F. Alic, A. Verberckmoes, P. Van Der Voort, J. Mater. Sci. 52, 628 (2017)
M.S. Kwon, N. Kim, C.M. Park, J.S. Lee, K.Y. Kang, J. Park, S.K. Min, N. Kim, M.P. Cheon, S.L. Jae, Y.K. Kyung, J. Park, Org. Lett. 7, 1077 (2005)
M. Veeranarayana Reddy, G. Chandra Sekhar Reddy, N. Thi Kim Lien, D. W. Kim, Y. T. Jeong, Tetrahedron 4 (2017)
S.R. Kanth, G.V. Reddy, P.H. Kishore, B.S. Rao, U.S.N. Murthy, Eur. J. Med. Chem. 41, 1011 (2006)
E. Scapin, C. P. Frizzo, L. V. Rodrigues, G. C. Zimmer, R. A. Vaucher, M. R. Sagrillo, J. L. Giongo, C. A. M. Afonso, P. Rijo, N. Zanatta, H. G. Bonacorso, M. A. P. Martins, Med. Chem. Res. 1 (2017)
R.K. Pavana, S. Choudhary, A. Bastian, M.A. Ihnat, R. Bai, E. Hamel, A. Gangjee, Bioorganic. Med. Chem. 25, 545 (2017)
K. Avasthil, N. Gargl, T. Chandral, D.S. Bhakunil, P.P. Gupta, R. C. Srimal. 1502, 585 (1993)
A. Haleel, D. Mahendiran, V. Veena, N. Sakthivel, A.K. Rahiman, Mater. Sci. Eng. C 68, 366 (2016)
A.Y. Kots, B.-K. Choi, M.E. Estrella-Jimenez, C.A. Warren, S.R. Gilbertson, R.L. Guerrant, F. Murad, Proc. Natl. Acad. Sci. USA 105, 8440 (2008)
D.R. Huron, M.E. Gorre, A.J. Kraker, C.L. Sawyers, N. Rosen, M.M. Moasser, Clin. Cancer Res. 9, 1267 (2003)
A. Gangjee, O. Adair, S.F. Queener, J. Med. Chem. 42, 2447 (1999)
D.C. Kim, Y.R. Lee, B.-S. Yang, K.J. Shin, D.J. Kim, B.Y. Chung, K.H. Yoo, Eur. J. Med. Chem. 38, 525 (2003)
L. Le Corre, A.-L. Girard, J. Aubertin, F. Radvanyi, C. Benoist-Lasselin, A. Jonquoy, E. Mugniery, L. Legeai-Mallet, P. Busca, Y. Le Merrer, Org. Biomol. Chem. 8, 2164 (2010)
G.K. Verma, K. Raghuvanshi, R. Kumar, M.S. Singh, Tetrahedron Lett. 53, 399 (2012)
J. Rangel, C. Díaz-Uribe, A. Rodriguez-Serrano, X. Zarate, Y. Serge, W. Vallejo, M. Nogueras, J. Trilleras, J. Quiroga, J. Tatchen, J. Cobo, J. Mol. Struct. 1137, 431 (2017).
M. Fattahi, A. Davoodnia, M. Pordel, Russ. J. Gen. Chem. 87, 863 (2017)
R. Ghorbani-Vaghei, N. Sarmast, J. Mahmoodi, Appl. Organomet. Chem. 1 (2016)
R. Ghorbani-Vaghei, A. Shahriari, Z. Salimi, S. Hajinazari, RSC Adv. 5, 3665 (2015)
S. Jin, H. Zhang, K. Xu, X. Ye, Y. Zhang, Y. Fang, D. Wang, Polyhedron 95, 108 (2015)
J.W. Lee, J.M. Ko, J.D. Kim, Electrochim. Acta 85, 459 (2012)
A.M. Kirillov, Coord. Chem. Rev. 255, 1603 (2011)
D.M. Yufanyi, A.M. Ondoh, J. Foba-Tendo, K.J. Mbadcam, Am. J. Chem. 5, 1 (2015)
M.V. Reddy, N.T.K. Lien, G.C.S. Reddy, K.T. Lim, Y.T. Jeong, Green Chem. 18, 4228 (2016)
J. Plank, Z. Dai, P.R. Andres, Mater. Lett. 60, 3614 (2006)
Acknowledgements
The authors wish to thank the Research Council of Bu-Ali Sina University for financial support to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghorbani-Vaghei, R., Sarmast, N. Hexamethylenetetramine grafted layered double hydroxides as a novel and green heterogeneous ionic liquid catalyst for the synthesis of pyrido[2,3-d]pyrimidine derivatives. Res Chem Intermed 44, 4483–4501 (2018). https://doi.org/10.1007/s11164-018-3399-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3399-8