Abstract
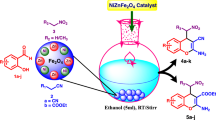
Magnetite (Fe3O4)-supported molybdenum oxide (MoO3) was synthesized from simple starting precursors in aqueous medium. The synthesized nanocat-Fe-Mo was analyzed using several techniques such as X-ray diffraction (XRD) analysis, X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and vibrating-sample magnetometry (VSM). The catalytic activity of the synthesized nanocat-Fe-Mo was studied in a benign one-pot multicomponent transformation for synthesis of amidoalkyl naphthol derivatives under solvent-free condition using both conventional and microwave irradiation methods. Nanocat-Fe-Mo was found to be highly active and could be reused seven times without notable loss in catalytic activity. The proposed method offers advantages such as good reaction yield (80–95%), short process time, simple workup, and recycling of the catalyst, representing important green chemistry principles.
Graphical Abstract










Similar content being viewed by others
Abbreviations
- Ar:
-
Aryl
- CCD:
-
Charge coupled device
- DMF:
-
Dimethyl formamide
- DMSO:
-
Dimethylsulfoxide
- EDS:
-
Energy dispersive spectroscopy
- Fe-Mo:
-
Ferrite-molybdenum
- FT-IR:
-
Fourier-transform infrared
- FWHM:
-
Full width at half maximum
- HIV:
-
Human immunodeficiency virus
- ICP-AES:
-
Inductively coupled plasma-atomic emission spectroscopy
- LFD:
-
Large field detector
- MNPs:
-
Magnetic nanoparticles
- MP:
-
Melting point
- MW:
-
Microwave
- NMR:
-
Nuclear magnetic resonance
- NP:
-
Nanoparticle
- Ph:
-
Phenyl
- ppm:
-
Parts per million
- RBF:
-
Round-bottomed flask
- RF:
-
Radiofrequency
- RT:
-
Room temperature
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
- TLC:
-
Thin-layer chromatography
- TMS:
-
Tetramethylsilane
- UV:
-
Ultraviolet
- VSM:
-
Vibrating-sample magnetometry
- XRD:
-
X-ray diffraction
References
G. Spada, E. Gavini, M. Cossu, G. Rassu, P. Giunchedi, Nanotechnology 23(9), 095101 (2012)
T.L. Doane, C. Burda, Chem. Soc. Rev. 41, 2885 (2012)
V. Berry, R.F. Saraf, Angew. Chem. Int. Ed. 44, 6668 (2005)
A. Schatz, T.R. Long, R.N. Grass, W.J. Stark, P.R. Hanson, O. Reiser, Adv. Funct. Mater. 20, 4323 (2010)
M.B. Gawande, S.N. Shelke, A.K. Rathi, P.S. Branco, R.K. Pandey, Appl. Organometal. Chem. 26, 395 (2012)
A. Baiker, Chem. Rev. 99(2), 453 (1999)
M.B. Gawande, P.S. Branco, R.S. Varma, Chem. Soc. Rev. 42, 3371 (2013)
B.R. Vaddula, A. Saha, R.S. Varma, Green Chem. 14(8), 2133 (2012)
S. Sa, M.B. Gawande, A. Velhinho, J.P. Veiga, R.S. Varma, P.S. Branco, Green Chem. 16, 3494 (2014)
M.B. Gawande, P.S. Branco, I.D. Nogueira, N. Bundaleski, O. Teodoro, R. Luque, Green Chem. 15(3), 682 (2013)
K. Routray, W. Zhou, C.J. Kiely, I.E. Wachs, J. Catal. 275, 84 (2010)
R. Luque, B. Baruwati, R.S. Varma, Green Chem. 12, 1540 (2010)
M.B. Gawande, S.N. Shelke, R. Zboril, R.S. Varma, Acc. Chem. Res. 47(4), 1338 (2014)
V. Polshettiwar, R.S. Varma, Chem. Soc. Rev. 37, 1546 (2008)
V. Polshettiwar, R.S. Varma, Acc. Chem. Res. 41, 629 (2008)
V.V. Namboodiri, R.S. Varma, Org. Lett. 4, 3161 (2002)
R.S. Varma, Appl. Sci. 4, 493 (2014)
C.R. Strauss, R.S. Varma, Microwaves in Green and Sustainable Chemistry, vol. 266 (Springer, Heidelberg, 2006), p. 199
A. Bruckmann, A. Krebs, C. Bolm, Green Chem. 10, 1131 (2008)
R.S. Varma, ACS Sustain. Chem. Eng. 4, 5866 (2016)
A. Hasaninejad, A. Zare, M. Shekouhy, J. Ameri-Rad, Green Chem. 13, 958 (2011)
M.B. Gawande, R.V. Jayaram, Catal. Commun. 7, 931 (2006)
S. Rana, S. Maddila, K. Yalagala, S.B. Jonnalagadda, Chem. Pub. Soc. 4, 703 (2015)
M.J. Aliaga, D.J. Ramon, M. Yus, Org. Biomol. Chem. 8, 43 (2010)
D.J. Ramon, M. Yus, Angew. Chem. Int. Ed. 44, 1602 (2005)
P.P. Ghosh, A.R. Das, Tetrahedron Lett. 53(25), 3140 (2012)
S. Knapp, Chem. Rev. 95, 1859 (1995)
D. Seebach, J.L. Matthews, Chem. Commun. 21, 2015(1997)
I. Szatmar, F. Fluop, Curr. Org. Synth. 1, 155 (2004)
A.Y. Shen, C.T. Tsai, C.L. Chen, Eur. J. Med. Chem. 34, 877 (1999)
N.P. Selvam, P.T. Perumal, Tetrahedron Lett. 47, 7481 (2006)
B. Das, B. Ravikanth, R. Rao, J. Mol. Catal. A: Chem. 261, 180 (2007)
Q. Zhang, J. Luo, Y. Wei, Green Chem. 12, 2246 (2010)
R.K. Singh, R. Bala, S. Kumar, Indian J Chem. B. 55, 381 (2016)
A.R. Hajipour, Y. Ghayeb, A.E. Ruoho, Tetrahedron Lett. 50, 7220 (2009)
K.C. Ashalu, J.N. Rao, J. Chem. Pharm. Res. 5(2), 44–47 (2013)
S. Kantevari, L. Nagarapu, Catal. Commun. 8, 1857 (2007)
G.H. Mahdavinia, M.A. Bigdeli, M.M. Heravi, Chin. Chem. Lett. 19, 1171 (2008)
L. Nagarapu, M. Baseeruddin, S. Apuri, S. Kantevari, Catal. Commun. 8, 1729 (2007)
S.N. Shelke, S.R. Bankar, M.B. Gawande, ACS Sustain. Chem. Eng. 2, 1699 (2014)
M.B. Gawande, V.D.B. Bonifacio, R.S. Varma, I.D. Nogueira, N. Bundaleski, C.A.A. Ghumman, O.M.N.D. Teodoro, P.S. Branco, Green Chem. 15, 1226 (2013)
M.B. Gawande, A.K. Rathi, I.D. Nogueira, R.S. Varma, P.S. Branco, Green Chem. 15(7), 1895 (2013)
T. Weber, J.C. Muijsers, J.W. Niemantsverdriet, J. Phys. Chem. 100, 14144 (1996)
D.O. Scanlon, G.W. Watson, D.J. Payne, G.R. Atkinson, R.G. Egdell, D.S. Law, J. Phys. Chem. C 114, 4636 (2010)
H.M. Lu, W.T. Zheng, Q. Jiang, J. Phys. D Appl. Phys. 40, 320 (2007)
M.A. Zolfigol, A. Khazaei, A. Zare, V. Khakyzadeh, Appl. Catal. A: Gen. 400, 70 (2011)
A.R. Supale, G.S. Gokavi, J. Chem. Sci. 122(2), 189 (2010)
A. Khazaei, M.A. Zolfigol, A.R. Zare, A. Parhami, A. Nezhad, Appl. Catal. A Gen. 386, 179 (2010)
M.M. Khodaei, A.R. Khosropour, H. Moghanian, Synlett 6, 916 (2006)
H. Moghanian, A. Mobinikhaledi, A.G. Blackman, E. Sarough-Farahani, RSC Adv. 4, 28176 (2014)
Z. Nasresfahani, M.Z. Kassaee, E. Eidi, New J. Chem. 40, 4720 (2016)
Acknowledgements
S.R.B. gratefully thanks the BCUD, Savitribai Phule Pune University, Pune for granting research stipend. The authors are grateful to the Director of SAIF, Panjab University (Chandigarh, India) for spectral analysis. S.R.B. thanks Dr. M. B. Gawande for constant inspiration. We also acknowledge the HOD and Principal, S.S.G.M. College, Kopargaon, Ahmednagar (MH) for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bankar, S.R., Shelke, S.N. Nanomagnetite-supported molybdenum oxide (nanocat-Fe-Mo): an efficient green catalyst for multicomponent synthesis of amidoalkyl naphthols. Res Chem Intermed 44, 3507–3521 (2018). https://doi.org/10.1007/s11164-018-3321-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3321-4